Abstract
Three new polymeric frameworks, [Ni(bbbm)(L1)2] n (1), [Ni(bbbm)(L2)2] n (2), and {[Co(bbbm)(L3)]·H2O} n (3) (bbbm = 1,4-bis(N-benzimidazolyl) butane, HL1 = 4-bromobenzoic acid, HL2 = 3-methylbenzoic acid, and H2L3 = glutaric acid) have been hydrothermally synthesized and structurally characterized by single-crystal X-ray diffraction. Complex 1 features a one-dimensional (1D) linear chain structure bridged by bbbm ligands, which is further connected into a supramolecular double chain structure through intermolecular π–π stacking interactions. Complex 2 contains 1D zigzag chain, which is further arranged into a 2D supramolecular architecture by hydrogen bonding and π–π stacking interactions. In the structure of 3, there are infinite 1D zigzag Co(II)-bbbm chains linked together by L3 ligands to generate an undulated 2D (4,4) sheet, which is further connected by intermolecular π–π stacking interactions to form a 3D supramolecular network. Furthermore, thermal stability and luminescent property of 1–3 were investigated.
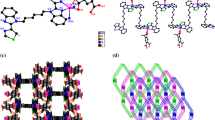
Graphical Abstract
Three new two/three-dimensional supramolecular complexes [Ni(bbbm)(L1)2] n (1), [Ni(bbbm)(L2)2] n (2), and {[Co(bbbm)(L3)]·H2O} n (3) [bbbm = 1,4-bis(N-benzimidazolyl) butane] have been synthesized from hydrothermal reaction of a metal salt with ligands: bbbm and one carboxylate ligand, 4-bromobenzoic acid (HL1), 3-methylbenzoic (HL2), and glutaric acid (H2L3).

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The assembly of metal–organic coordination polymers has been a rapidly expanding research field [1–5]. This is due to their potential as functional materials with specific properties, such as luminescence, magnetism, absorption, catalysis, ion-exchange, etc. [6–11]. To date, the rational and controllable synthesis of coordination networks and supramolecular architectures is still a difficult challenge in most cases. Several factors, such as π–π stacking and H-bonding interactions etc., may affect assembly of the compounds [12, 13]. Thus, a key step for the construction of such complexes is to select suitable functional ligands which possess strong coordination ability and can provide the hydrogen bond acceptors/donors and π-conjugated system for extending their networks [14–16].
On the basis of the aforementioned points, we select flexible multidentate ligand 1,4-bis(N-benzimidazolyl) butane (bbbm) with aliphatic core and benzimidazole nitrogen donors as main ligand because of its remarkable advantages (Scheme 1): (i) the bis(benzimidazole) nitrogens have strong coordination ability and the –(CH2) n – group is flexible [17–19]; (ii) bbbm ligand contains both an imidazole ring and a larger conjugated π-system, capable of acting as hydrogen bond donors and for π–π stacking interactions. For example, Lang and co-workers have designed and synthesized a series of coordination polymers based on a series of flexible bidentate benzimidazole-containing organic ligands [20]. Hou and co-workers have also reported some coordination polymers based on flexible bbbm ligand [21–24]. To our knowledge, the reports on the bbbm-based compounds with organic carboxylates as the secondary ligands are relatively limited.
Here, we select three structurally representative organic carboxylate ligands 4-bromobenzoic acid (HL1), 3-methylbenzoic acid (HL2), and glutaric acid (H2L3) acting as secondary ligands. Three new coordination polymers [Ni(bbbm)(L1)2] n (1), [Ni(bbbm)(L2)2] n (2), and {[Co(bbbm)(L3)]·H2O} n (3) have been successfully synthesized under hydrothermal conditions. In addition, the thermal stability and luminescent property of 1–3 have also been investigated in the solid state.
Experimental Procedures
Materials and Methods
Solvents and starting materials for synthesis were commercially available and used as received. Ligand bbbm was prepared according to the literature [25]. FT-IR spectra (KBr pellets) were taken on a Magna FT-IR 560 spectrometer. Thermogravimetric data were performed using a Pyris Diamond thermal analyzer. Elemental analyses (C, H, and N) were performed on a Perkin-Elmer 240C analyzer. Fluorescence spectra were recorded at room temperature on a Hitachi F-4500 fluorescence/phosphorescence spectrophotometer.
Synthesis of [Ni(bbbm)(L1)2] n (1)
A mixture of Ni(NO3)2·6H2O (0.029 g, 0.1 mmol), HL1 (0.04 g, 0.2 mmol) and bbbm (0.015 g, 0.05 mmol) was dissolved in 8 mL of distilled water and stirred for 0.5 h. Then the pH was adjusted to 6.0 by the addition of 0.1 M NaOH solution. Consequently, the resulting mixture was transferred and sealed in a 25 mL Teflon-lined stainless steel vessel, which was heated at 150 °C for 50 h, and then, the reaction system was cooled to room temperature at a rate of 5 °C h−1. Green block crystals of 1 suitable for X-ray diffraction were isolated by mechanical separation from amorphous solid in 28% yield (based on Ni(II) salt). Anal. Calcd for C32H26Br2NiN4O4: C 51.26, H 3.47, N 7.48%. Found: C 51.46, H 3.43, N 7.54%. IR (KBr, cm−1): 3101 (m), 3060 (w), 2945 (w), 2360 (s), 1587 (s), 1533 (s), 1508 (w), 1461 (s), 1421 (s), 1294 (m), 1245 (s), 1201 (s), 856 (s), 773 (s), 748 (s), 671 (m).
Synthesis of [Ni(bbbm)(L2)2] n (2)
The synthetic procedure for 2 is the same as that for 1 except that HL2 (0.027 g, 0.2 mmol) was used instead of HL1. Green crystals of 2 suitable for X-ray diffraction were isolated by mechanical separation from amorphous solid in 31% yield (based on Ni(II) salt). Anal. Calcd for C34H32NiN4O4: C 65.88, H 5.17, N 9.04%. Found: C 65.69, H 5.13, N 9.11%. IR (KBr, cm−1): 3114 (w), 3058 (w), 2923 (w), 2360 (s), 1593 (w), 1525 (s), 1440 (s), 1392 (s), 1296 (m), 1263 (w), 1220 (s), 808 (m), 761 (s), 742 (s), 675 (m).
Synthesis of {[Co(bbbm)(L3)]·H2O} n (3)
The synthetic procedure for 3 is the same as that for 1 except that H2L3 (0.013 g, 0.1 mmol) and CoCl2·6H2O (0.024 g, 0.1 mmol) were used instead of HL1 and Ni(NO3)2·6H2O. Purple-red crystals of 3 suitable for X-ray diffraction were isolated by mechanical separation from amorphous solid in 45% yield (based on Co(II) salt). Anal. Calcd for C23H26CoN4O5: C 55.49, H 5.23, N 11.26%. Found: C 55.65, H 5.21, N 11.34%. IR (KBr, cm−1): 3329 (m), 3095 (m), 3002 (w), 2937 (w), 2360 (s), 1637 (m), 1541 (s), 1509 (s), 1429 (m), 1396 (s), 1298 (s), 1254 (s), 1206 (m), 757 (s), 740 (s), 668 (m).
X-ray Crystallographic Study
A Bruker Apex CCD diffractometer (Mo-Kα radiation, graphite monochromator, λ = 0.71073 Å) was used to collect data. The structures were solved by direct methods with SHELXS-97 and Fourier techniques and refined by the full-matrix least-squares method on F 2 with SHELXL-97 [26, 27]. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms of ligands were generated theoretically onto the specific atoms and refined isotropically with fixed thermal factors. The H-atoms of water molecules have not been localized. All the crystal data and structure refinement details for the three complexes are given in Table 1. The data of relevant bond distances and angles are listed in Table 2.
Results and Discussion
Description of the Structure
[Ni(bbbm)(L1) 2 ] n ( 1 ) The asymmetric unit of 1 consists of one bbbm, two L1 ligands, one Ni(II) ion. As displayed in Fig. 1, each Ni(II) ion with a distorted octahedral coordination geometry is coordinated by four oxygen atoms (O1, O2, O1A, O2A) from two distinct L1 ligands and two N atoms (N1, N1A) from two different bbbm ligands. The N1A, O1, O2, and O2A atoms comprise the equatorial plane, and the axial positions are occupied by N1 and O1A. The Ni–O distances range from 2.052(2) to 2.215(2) Å, and the Ni–N distance is 2.040(3) Å (Table 2).
The Ni(II) ions are linked into one-dimensional (1D) linear chain [Ni(bbbm)] through bridging bbbm ligands. The crossed L1 ligands are attached to one side of 1D linear chain. Interestingly, the two adjacent linear chains are further connected into a supramolecular double chain through intermolecular π–π stacking interactions between the aromatic ring of the carboxylate ligand and the benzimidazole ring of the neighboring chain (3.987 Å) (Fig. 2).
[Ni(bbbm)(L2) 2 ] n ( 2 ) The structure of 2 is a 1D zigzag chain. The asymmetric unit of 2 is composed of one Ni(II) ion, one bbbm ligand, and two L2 ligands (Fig. 3). Each Ni(II) ion is six-coordinated by two nitrogen atoms from two bbbm ligands and four oxygen atoms from two L2 ligands, showing a distorted octahedral geometry. The Ni–O bond lengths range from 2.047(3) to 2.242(3) Å, and the Ni–N bond lengths are 2.011(4) and 2.046(4) Å, respectively. Each Ni(II) is linked to adjacent Ni(II) through the bridging bbbm, forming a 1D zigzag chain structure (Fig. 4a), where the Ni–Ni–Ni angle, defined by the orientation of the bbbm ligands in the chain, is 101.51°. To be noticed, the L2 ligands in 2 are arranged at both sides of the chain.
In addition, the adjacent 1D chains are further arranged into a 2D supramolecular architecture by the intermolecular π–π stacking interactions between the aromatic ring of the carboxylate ligand and the benzimidazole ring of the neighboring chain (3.975 Å) and one kind of hydrogen bonding interactions [C(8)–H(8A)···O(4) 3.1808 Å, 134º and C(18)–H(18A)···O(2) 3.1079 Å, 121º] (Fig. 4b). The weak noncovalent interactions are important in the formation of the final supramolecular structure of 2.
{[Co(bbbm)(L3)]·H 2 O} n ( 3 ) The fundamental building unit of 3 contains one cobalt ion, one bbbm ligand, one L3 ligand and one interstitial water molecule. Each Co(II) cation exhibits a distorted trigonal–bipyramidal geometry with a CoN2O3 coordination environment, and is coordinated by three oxygen atoms from two L3 ligands and two nitrogen atoms from two bbbm ligands, as shown in Fig. 5. The Co–O bond lengths in 3 are in the range of 2.023(4) to 2.392(4) Å, and the Co–N distances are 2.033(4) and 2.076(3) Å, which is comparable with those observed in other cobalt(II)-containing complexes [28].
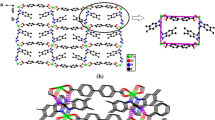
Each bbbm ligand links two Co(II) ions in 3 to form an infinite 1D zigzag chain. The adjacent Co-bbbm chains are further linked by L3 ligands to give an undulated 2D (4,4) sheet (Fig. 6a, b). Each L3 ligand shows both a monodentate and chelating coordination mode. The Co···Co distances separated by bbbm and L3 are 13.916, 13.646, and 8.750 Å, respectively.
Furthermore, a kind of π–π interactions between benzimidazole rings in 3 (centroid-to-centroid distances of 3.983 and 3.714 Å) consolidate the overall structure. The adjacent 2D architectures are further connected by the intermolecular π–π stacking interactions between benzimidazole ring of bbbm ligand and the benzimidazole ring of the neighboring 2D layer to form a 3D supramolecular network (Fig. 7).
In the previously reported related complexes, a 2D [(CuSCN)4(bbbm)3] n with a kind of [CuSCN] n double chain has been reported by Lang et al. [20]. One 3D {[Cd(bbbm)(SO4)(H2O)2]CH3OH} n and two 2D {[Ni(bbbm)2(H2O)2](NO3)2·2CH3OH·6H2O} n and {[Co(bbbm)2(H2O)2](NO3)2·2CH3OH·6H2O} n have been reported by Hou et al. [23]. To our knowledge, the reports on the bbbm-based compounds with organic carboxylates as the secondary ligands are relatively limited. In this work, we selected flexible bis(benzimidazole) bbbm as the main ligand and different carboxylate ligands as the secondary ligands, intending to observe carboxylate ligand effect on the assembly of the coordination polymers. From the structures of 1–3, it can be seen that the different carboxylate ligands result in the corresponding structural changes. The different 1D linear/zigzag chain structures of 1 and 2 are formed, and the 2D sheet structure of 3 is obtained. Complexes 1, 2 and 3 are ultimately extended to supramolecular double chain, 2D and 3D networks by hydrogen bonding and π–π stacking interactions, which is different from the above reported complexes. As can be seen from the description of structures, the bbbm can rotate and bend around the –CH2– group freely when coordinating to the central metals. Thus, different structures have been obtained with the change of conformations of bbbm ligands. As shown in Fig. 8, the flexible bbbm ligands adopt three conformations in compounds 1–3, [with M···M separation 11.487(5), 13.0688(30) and 13.9155(30) Å]. The C–C–C–C torsion angle for each compound is 79.013, 157.073 and 180.00. It is obvious that different conformations can lead to different lengths of the bbbm, which play important roles in the formation of different structures.
Thermal Stability Analysis
Thermogravimetric analyses (TGA) are performed to measure the thermal stabilities of 1–3 as shown in Fig. 9. In compounds 1 and 2, a rapid weight loss of 290–650 and 330–555 °C corresponds to the organic ligands and the remaining residue is NiO (calcd: 9.97%; found: 9.63% for 1; calcd: 12.06%; found: 12.16% for 2). Polymer 3 lost the interstitial water at 102–164 °C (calcd: 3.62%; found: 3.32%). Upon further heating, the organic mixed-ligands of 3 are observed to be decomposed progressively, leaving CoO as the final product (calcd: 15.06%; found: 14.95%).
Luminescent Property
The luminescent behaviors of polymers 1–3 and free ligand bbbm were studied in the solid state at room temperature (Fig. 10). The free ligand bbbm displays luminescence with emission band at 435 nm (λex = 312 nm). Meanwhile, the emission for the complexes 1–3 can be observed, where the emission bands appear at 368 nm (λex = 235 nm) for 1, 470 nm (λex = 250 nm) for 2 and 494 nm (λex = 324 nm) for 3. Compared with that of the free ligand bbbm, the emission peaks of 1–3 are 67 nm blue-shifted for 1, 35 nm red-shifted for 2 and 59 nm red-shifted for 3, which may be due to the increase of conjugation upon metal coordination and originate from the ligand-to-metal charge-transfer (LMCT) [29–32].
Conclusions
In this article, the assembly reactions of the flexible ligand bbbm and nickel(II) or cobalt(II) salts with auxiliary carboxylate ligands produce three coordination polymers [Ni(bbbm)(L1)2] n (1), [Ni(bbbm)(L2)2] n (2), and {[Co(bbbm)(L3)]·H2O} n (3) under hydrothermal conditions. Complexes 1 and 2 feature linear and zigzag chain structures, respectively. Complex 3 has an undulated 2D (4,4) network. Complexes 1–3 are further connected by intermolecular interactions to form supramolecular double chain, 2D and 3D supramolecular networks, respectively. The flexibility of bbbm, the diversity of the carboxylate and the different coordination preferences of the Co(II) and Ni(II) metal ions lead cooperatively to the different architectures from 1D chain to 2D layer.
Supplementary Material
CCDC 798761–798763 for complexes 1–3 contain the supplementary crystallographic data for this article. The data can be obtained free of charge at http://www.ccdc.cam.ac.uk/conts/retrieving.html [or from Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cambridge CB2, 1EZ, UK; fax: +44 (0) 1223-336033; e-mail: deposit@ccdc.cam.ac.uk].
References
Dan-Hardi M, Serre C (2009) J Am Chem Soc 131:10857
Eddaoudi M, Kim J, Rosi N, Vodak D, Wachter J, O’Keeffe M, Yaghi OM (2002) Science 295:469
Zou RQ, Sakurai H, Han S, Zhong RQ, Xu Q (2007) J Am Chem Soc 129:8402
Dan M, Rao CNR (2006) Angew Chem Int Ed 45:281
Robin AY, Fromm KM (2006) Coord Chem Rev 250:2127
Kaneko W, Ohba M, Kitagawa S (2007) J Am Chem Soc 129:13706
Cheng L, Zhang WX, Ye BH, Lin JB, Chen XM (2007) Eur J Inorg Chem 18:2668
Zhu XD, Lu J, Li XJ, Gao SY, Li GL, Xiao FX, Cao R (2008) Cryst Growth Des 8:1897
Yoon JW, Jhung SH, Hwang YK, Humphrey SM, Wood PT, Chang JS (2007) Adv Mater 19:1830
Chae HK, Siberio DY, Kim J, Eddaoudi GY, Matzger MJ, Keeffe MO, Yaghi OM (2004) Nature 427:523
Kitagawa S, Uemura K (2005) Chem Soc Rev 34:109
Wang XL, Chen YQ, Zhang JX, Liu GC, Lin HY, Tian AX (2010) Z Anorg Allg Chem 636:830
Wang LS, Zhu L, Yin PC, Wei YG (2009) Inorg Chem 48:9222
Yang J, Ma JF, Liu YY, Ma JC, Batten SR (2007) Inorg Chem 46:6542
Zhou LJ, Wang YY, Zhou CH, Wang CJ, Shi QZ, Peng SM (2007) Cryst Growth Des 7:300
Hunger J, Krautscheid H, Sieler J (2009) Cryst Growth Des 9:4613
Yang HX, Meng XR, Liu Y, Hou HW, Fan YT, Shen XQ (2008) J Solid State Chem 181:2178
Chen JQ, Cai YP, Fang HC, Zhou ZY, Zhan XL, Zhao G, Zhang Z (2009) Cryst Growth Des 9:1605
Li SL, Lan YQ, Ma JC, Ma JF, Su ZM (2010) Cryst Growth Des 10:1161
Li LL, Yuan RX, Liu LL, Ren ZG, Zheng AX, Cheng HJ, Li HX, Lang JP (2010) Cryst Growth Des 10:1929
Niu YY, Zhang N, Hou HW, Zhu Y, Tang MS, Ng SW (2007) J Mol Struct 827:195
Fan J, Hanson BE (2005) Inorg Chem 44:6998
Chang Q, Meng XR, Song YL, Hou HW (2005) Inorg Chim Acta 358:2117
Xiao B, Han HY, Meng XR, Song YL, Fan YT, Hou HW, Zhu Y (2004) Inorg Chem Commun 7:378
She JB, Zhang GF, Dou YL (2006) Acta Crystallogr Sect E E62:o402
Sheldrick GM (1997) SHELXS-97, program for the solution of crystal structure. University of Göttingen, Göttingen
Sheldrick GM (1997) SHELXL-97, program for the refinement of crystal structure. University of Göttingen, Göttingen
Yang YT, Luo F, Che YX, Zheng JM (2008) Cryst Growth Des 8:3508
Wang XL, Bi YF, Lin HY, Liu GC (2007) Cryst Growth Des 7:1086
Pan ZR, Song Y, Jiao Y, Fang ZJ, Li YZ, Zheng HG (2008) Inorg Chem 47:5162
Liu GX, Huang YQ, Chu Q, Okamura T, Sun WY, Liang H, Ueyama N (2008) Cryst Growth Des 8:3233
Zhou DS, Wang FK, Yang SY, Xie ZX, Huang RB (2009) CrystEngComm 11:2548
Acknowledgments
Financial supports of this research by the NCET-09-0853, Talent-supporting Program Foundation of Liaoning Province (No. 2009R03) and Program Foundation of Liaoning Province Silicon Materials Engineering Technology Research Center (2009402007) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, XL., Zhang, JX., Hou, LL. et al. Three New Complexes Based on a Flexible Bis(benzimidazole) Ligand and Rigid/Flexible Organic Carboxylates. J Chem Crystallogr 41, 1579–1585 (2011). https://doi.org/10.1007/s10870-011-0143-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-0143-2