Abstract
A novel 3,4,7,8-tetrachloro-1,10-phenanthroline (Cl4phen) Zn(II) complex has been synthesized. The complex, [Zn(Cl4phen)2(H2O)2](NO3)2·CH3CH2OH (1), has been identified and characterized by single crystal X-ray diffraction, elemental analysis, FT-IR, thermogravimetric analysis and photoluminescence studies. Single crystal X-ray diffraction analysis reveals that complex (1) belongs to the monoclinic system, space group P2(1)/c, a = 10.061(2) Å, b = 18.924(4) Å, c = 18.189(4) Å, β = 100.94(3)°, and Z = 4. Complex (1) consists of cationic species [Zn(Cl4phen)2(H2O)2]2+, NO3 − and CH3CH2OH. The zinc atom displays a distorted cis-N4O2 octahedral geometry. Via extended Zn–O–H···O–N–O···H–O–Zn bridge, every mononuclear unit is linked with other ones to form one-dimensional (1D) infinite chain of hydrogen bond system. Three-dimensional (3D) polymeric network arrangement was built via weak C–H···O and π-stacking interactions between Cl4phen moieties. A solvent-dependency effect of complex (1) was observed in spectroscopic properties.
Graphical Abstract
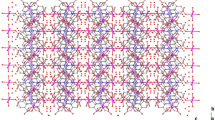
Three-dimensional (3D) polymeric network arrangement was built via weak C–H···O and π-stacking interactions between Cl4phen moieties.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their interesting biological activities, such as antibacterial [1], anti-convulsant [2] and antiproliferative–antitumor [3–5] activity, zinc(II) complexes had attracted great attention in the recent years. As drugs for the treatment of Alzheimer disease [6], zinc(II) complexes had been structurally characterized. Zinc(II) complexes had also showed properties of DNA binding in the very recent research [7]. Moreover, zinc(II) complexes with appropriate ligands can be applied in optical devices, such as OLED [8] and light switching device [9].
Behaving as σ(chelate) donor/π acceptor ligand, 1,10-phenanthroline with the low-lying π* orbitals is attractive to many chemists due to its tunability. Some research results indicate that the lowest-lying orbital of 1,10-phenanthroline depends on the substituent pattern of 1,10-phenanthroline [10, 11]. Several efforts, such as increasing the conjugation systerm of 1,10-phenanthroline [12] and extending the N-heterocyclic aromatic ring [13], have been reported to tune the physical and chemical properties of the 1,10-Phenanthroline π system through chemical modification. Multiple acceptor effect by perchlorination of 1,10-phenanthroline makes π* orbitals of octochloro-1,10-phenanthroline strongly stabilized with 1.69 eV lower than 1,10-phenanthroline itself [14]. Tetra-chloration of 1,10-phenanthroline can also lower the of π* orbitals to bring about some different photochemical and photophysical properties. Meanwhile, 1,10-phenanthroline moiety can efficiently provide π–π stacking interactions in either intra- or inter-molecular mode. This feature contributes the stability of structures as well as the formation of extended supramolecular structures [15]. Here, we report the synthesis, crystal structure and photoluminescence properties of zinc(II) complex (1). Complex (1) consist of [Zn(Cl4phen)2(H2O)2]2+ unit, one ethanol molecular and two non-coordinating nitrate radical anions in the unit cell. Adjacent units are linked by hydrogen bonds and π-stacking interactions to form three-dimensional structure.
Experimental
Materials and Physical Measurements
3,4,7,8-Tetrachloro-1,10-phenanthroline was prepared by the methods as reported earlier [16], then purified by recrystallization twice in DMF. All other chemicals were commercially available and used without further purification. Elemental analysis was performed on a Elementar Vario EL III elementer analyzer. IR spectra were obtained as KBr discs on an ABB Bomem FTLA 2000-104 spectrophotometer in the 4,000–500 cm−1 region. Thermal analysis (TG) measurements were performed using Mettler Toledo TGA/SDTA85/e Thermal Analyzer with heating rate of 20 °C/min. The fluorescent data were collected on a RF-5301 double beam spectrofluorometer at room temperature. The spectroscopic absorption spectra were scanned on a TU-1901 double beam ultraviolet-visible spectrophotometer. 1H-NMR spectra were scanned with a Bruker AMX400 spectrometer.
Preparation of [Zn(Cl4phen)2(H2O)2](NO3)2·CH3CH2OH (1)
To an solution (20 mL) of zinc nitrate (3.0 g, 10 mmol) in ethanol was added an aqueous solution (10 mL) of 3,4,7,8-tetrachloro-1,10-phenanthroline (2.0 g, 10 mmol) in CHCl3 with stirring. The mixture was heated to reflux and stirred for 2 h. Crystalline solid was obtained by slow evaporation of the solvent. Yield: 4.1 g, 90%. Anal. Calcd. for C26H18Cl8N6O9Zn (mol. wt. 907.43): C, 34.40; H, 1.98; N, 9.26. Found: C, 34.62; H, 1.90; N, 9.18. IR (cm−1): 3,441(br, m), 1,611(w), 1,576(w), 1,499(w), 1,416(m), 1,384(s), 1,327(m), 1,203(w), 845(w), 824(w). 1H-NMR (DMSO-d 6): δ 8.285(s, 4 H, phen-H); 8.023(d, 4 H, phen-H).
X-ray Data Collection and Structural Determination
The diffraction data is collected on a Bruker Smart AP EX II CCD diffractionmeter with graphite monochromated Mo–Ka radiation (λ = 0.71073Å) at 273 K. Absorption corrections are applied by SADABS. The structure was solved by direct methods and refined with full-matrix least-squares technique using SHELXTL 97 [17]. All nonhydrogen atoms were refined with anisotropic displacement parameters. The crystallographic details and selected bond lengths (Å) and bond angles (°) are shown in Tables 2 and 3.
Result and Discussion
1H-NMR, IR Spectra and Thermal Analysis
The 1H-NMR spectra show that the signals of aromatic protons in complex (1) shift to upper field (lower δ values), compared to the free ligand. It indicates that complex (1) is diamagnetic similar as the reported six-coordinated Zn(II) complex [18].
The IR spectra of the complex show a broad peak at 3,441 cm−1 assignable to σ(OH) stretching vibrations of ethanol molecular and coordinated water molecules [19]. The observed position of the σ (OH) bands is in agreement with the participation of water molecules and ethanol molecular in hydrogen bonds. The strong absorption band at 1,384 cm−1 is attributed to non-coordinated NO3 − [20] in accordance with the result of the X-ray analysis detailed below.
The thermal gravimetric (TG) curve of (1) is shown in Fig. 1. The total weight loss of 85.12% (calculated value: 84.55%) in the range of 25–697 °C agrees with the release of two ligands, two water, ethanol and nitrate anions. The remainder of 14.88% corresponds to Zn and two Cl atoms (calculated value: 15.02%) of total weight. Decomposition of complex (1) starts at 97 °C. There is a weight loss of 4.6% at 121 °C corresponding to the loss of non-coordinated ethanol molecule (calcd. 5.07%). The following weight loss of 4.44% at 229 °C may attribute to the loss of two coordinated water molecules (calcd. 4.09%). With the loss of two Cl4-phen moieties, complex (1) decompose slowly in the temperature range of 229–697 °C, in concomitance with the reduction of nitrate anions. The residue is in 14.88% of total weight, consistent with the weight of Zn and two Cl atoms in complex (1). As illustrated in Fig. 1, the complex (1) seems less stable than the reported [Zn(H2O)4(phen)](NO3)2·H2O(ca.320 °C) [21].
Spectroscopic Absorption and Photoluminescence Properties
Spectroscopic absorption spectra of complex (1) in DMSO and Ethanol/dichloromethane (1:1) are shown in Fig. 2. The position of the electronic absorption maxima and the extinction coefficients for complex (1) are given in Table 1. Complex (1) has two absorption bands at about 250 and 350 nm, attributed to π → π* and n → π* electron transition, respectively.
Excitation of LC (ligand center, n → π*) bands of complex (1) at room temperature produced emission at longer wavelength. The emission spectra of complex (1) are showed in Fig. 3 and the position bands are included in Table 1. The intensity of emission peak at 458 nm in DMSO is much stronger than that at 423 nm in Ethanol/dichloromethane (1:1). It reveals that complex (1) show solvent-dependency in the spectroscopic properties.
Descriptions of the Structure
Crystallographic data for the structure of complex (1) are listed in Table 2. The zinc complex (1) is illustrated in Fig. 4. The zinc center displays a distorted octahedral geometry with the major distortion, which is manifested in the chelate angle formed by the Cl4phen ligand, i.e. 76.92(1)°. The conformation about the zinc atom is described as sy-cis-configuration. Selected bond distances and angles are summarized in Table 3. The average bond length of Zn–N is comparable to some other six-coordinate zinc complexes, e.g. [Zn(phen)2(H2O)2]2+ (2.17(1) Å)[22] and [Zn(H2O)4(phen)]2+ (2.113(3) Å, 2.127(3) Å) [23]. The bond angles of N(1)–Zn(1)–N(2) 76.82(10)°, N(4)–Zn(1)–N(3) 76.71(1)° in (1) are similar to those in [Zn(phen)2(H2O)2]2+(average 76.8(1)°), but a bit smaller than the reported in [Zn(H2O)4(phen)]2+ 79.34(1)°. The ligand (Cl4phen) shows a slightly twisted conformation in Zn(Cl4phen)2(H2O) 2+2 , in which four chlorine atoms at 3,4,7,8-positions and two hydrogen atoms at 5,6-positions deviate from planarity. The deviation from planarity has also been observed in Ag(ocp) +2 (ocp = octachloro-1,10-phenanthroline) by Christoph Titze et al. [14]. And the appropriate reasons for the deviation from planarity are the nonbonded interactions between chloride atoms.
Meanwhile, there are a large number of intra- and inter-molecular O–H···O and weak C–H···O hydrogen bonds in the crystal structure of complex (1). Two oxygen atoms of nitrate radical anion (O6 and O7) are hydrogen bonded to two hydrogen atoms (H28 and H29) of chelating water molecule from two different units. Every mononuclear unit is linked to other ones via extended Zn–O–H···O–N–O···H–O–Zn bridge to form one-dimensional (1D) infinite chain of hydrogen bond system (Fig. 5). The distances of H···O in O2–H29···O6 and O1–H28···O7 are 2.699 and 2.743 Å, respectively. Then, two 1D infinite chains were linked by the other free nitrate radical anion to form 2D network. Two oxygen atoms (O5 and O3) of nitrate radical anion form weak C–H···O hydrogen bonds with two hydrogen atoms of Cl4phen at 5,6-positions, with the bond length (H···O) 3.209 and 3.300 Å, respectively. Meantime, O5 forms hydrogen bond with H27 as well, with the bond length 2.741 Å (Table 4). The phenanthroline moieties of the neighboring infinite chains are parallel with each other (Fig. 6). The minimum distance between two phenanthroline moieties is ~3.455(1) Å. It indicates that the neighboring units are connected via π-stacking interactions. Similar interactions are observed in zinc hydrogen aconitate 1D polymers [24], in which the distance between the least square planes is 3.496 Å. The strong O–H···O hydrogen bonds, weak close C–H···O contacts and π-stacking interactions stabilize the structure to form the three-dimensional (3D) network as shown in Fig. 6.
Conclusions
The new Zn(II) complex [Zn(Cl4phen)2(H2O)2](NO3)2·CH3CH2OH (1) has been obtained and characterized by single crystal X-ray diffraction. The data of crystal structure reveal that multidimensional supramolecular structure has assembled via O–H···O and weak C–H···O hydrogen bonds, and π-stacking interactions. All these interactions have played a significant role in the crystal structure. The result of spectroscopic absorption and photoluminescence suggest that complex (1) has a solvent-dependency effect.
Supplementary Material
CCDC-69984 contains the supplementary crystallo-graphic data for this paper. These data can be obtained free of charge at http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 (0)1223-336033; email: deposit@ccdc.cam.ac.uk).
References
Li ZQ, Wu FJ, Gong Y, Hu CW, Zhang YH, Gan MY (2007) Chin J Chem 25:1809
d’Angelo J, Morgant G, Ghermani NE, Desmaele D, Fraisse B, Bonhomme F, Dichi E, Sghaier M, Li Y, Journaux Y, Sorenson JRJ (2008) Polyhedron 27:537
Kovala-Demertzi D, Yadav PN, Wiecek J, Skoulika S, Varadinova T, Demertzis MA (2006) J Inorg Biochem 100:1558
Travnicek Z, Krystof V, Sipl M (2006) J Inorg Biochem 100:214
Casas JS, Castellano EE, Couce MD, Ellena J, Sanchez A, Sordo J, Taboada C (2006) J Inorg Biochem 100:124
Di Vaira M, Bazzicalupi C, Orioli P, Messori L, Bruni B, Zatta P (2004) Inorg Chem 43:3795
Tarushi A, Psomas G, Raptopoulou CP, Kessissoglou DP (2009) J Inorg Biochem 103:898
Chen L, Qiao J, Xie JF, Duan L, Zhang DQ, Wang LD, Qiu Y (2009) Inorg Chim Acta 362:2327
Ngan TW, Ko CC, Zhu NY, Yam WW (2007) Inorg Chem 46:1144
Klein A, Kaim W, Waldhör E, Hausen HD (1995) J Chem Soc Perkin Trans 2:2121
Ernst S, Vogler C, Klein A, Kaim W, Zalis S (1996) Inorg Chem 35:1295
Joshi HS, Jamshidi R, Yitzhak T (1999) Angew Chem Int Ed 38:2721
Delaney S, Pascaly M, Bhattacharya PK, Han K, Barton JK (2002) Inorg Chem 41:1996
Titze C, Kaim W (1997) Inorg Chem 36:2505
Xu XX, Lu Y, Wang EB, Ma Y, Bai XL (2007) Inorg Chim Acta 360:455
Yamada MA, Nakamura Y, Hasegawa IA et al (1992) Bull Chem Soc Jpn 65:2007
Sheldrick GM (1997) SHELX-97 Structure Solution and Refinement Package. University of Gottingen, Germany
Arif M, Nazir S, Iqbal MS, Anjumc S (2009) Inorg Chim Acta 362:1624
Chen XF, Cheng P, Liu X, Zhao B, Liao DZ, Yan SP, Jiang ZH (2001) Inorg Chem 40:2652
Curtis NF, Curtis YM (1965) Inorg Chem 4:804
Schäfer B, Görls H, Meyer S, Henry W, Johannes GV, Rau S (2007) Eur J Inorg Chem 25:4056
Mahata P, Natarajan S (2005) Eur J Inorg Chem 11:2156
Zhang CG, Janiak C (2001) J Chem Cryst 31:29
George E, Kostakis NordlanderE, Hadjiliadis N, Haukka M, Plakatouras JC (2006) Inorg Chem Comm 9:915
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, CG., Qian, QL., Liu, YF. et al. Synthesis, Crystal Structure and Characterizations of New 3,4,7,8-Tetrachloro-1,10-Phenanthroline Zn(II) Complex. J Chem Crystallogr 40, 19–24 (2010). https://doi.org/10.1007/s10870-009-9598-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9598-9