Abstract
Pyrroline-5-carboxylate reductase 1 (PYCR1) plays a significant role in the malignant progression of various cancers. However, the role of PYCR1 in bladder cancer has not been well studied. This study was performed to evaluate the potential relevance of PYCR1 in bladder cancer. Our data revealed that PYCR1 expression was increased in bladder cancer tissues, and increased expression of PYCR1 was predictive of decreased survival rates. In bladder cancer cell lines, knockdown of PYCR1 caused significantly retarded cell growth and invasion, while PYCR1 overexpression accelerated cellular proliferation and invasion. Moreover, PYCR1 knockdown decreased levels of phosphorylated Akt, and enhanced activation of Wnt/β-catenin signaling. Akt inhibition markedly abrogated of PYCR1 overexpression-mediated activation of Wnt/β-catenin signaling. In addition, overexpression of β-catenin partially reversed PYCR1 knockdown-mediated tumor suppression. Notably, PYCR1 knockdown significantly impeded tumor formation and growth in bladder cancer cells in vivo. In conclusion, these data demonstrate that PYCR1 is highly expressed in bladder cancer and knockdown of PYCR1 exerts a remarkable inhibitory effect on tumor formation via downregulation of Akt/Wnt/β-catenin signaling. Our study suggests a potential role for PYCR1 in promoting bladder cancer progression and indicates that PYCR1 may be utilized as an attractive and promising anticancer target for treatment of bladder cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is one of the most common urinary malignant tumors and is currently a leading cause of cancer-associated death worldwide (Kamat et al. 2016; Bray et al. 2018). In particular, bladder cancer morbidity and mortality rates in China have been rapidly increasing over the past few decades (Pang et al. 2016). Despite improvements in current therapies for bladder cancer, bladder cancer patient outcomes remain poor (Witjes et al. 2014; Berdik 2017). The pathogenesis of bladder cancer is complicated, and involves various genetic alterations and dysregulation of multiple signaling pathways (McConkey et al. 2010). However, the precise underlying molecular mechanisms remain elusive. Elucidation of the molecular mechanisms responsible for the development and progression of bladder cancer may facilitate the development of promising treatments for the disease.
Pyrroline-5-carboxylate reductase 1 (PYCR1) is a member of PYCR family that plays a pivotal role in regulating proline synthesis (Adams and Frank 1980; De Ingeniis et al. 2012). PYCR1 is primarily localized within the mitochondria and is involved in regulating oxidative stress (Yasuda et al. 2013). Mutation of PYCR1 is associated with the development of autosomal recessive cutis laxa (Reversade et al. 2009). Particularly, PYCR1 acts as a key modulator of the development and progression of multiple cancers. High levels of expression of PYCR1 have been detected in various tumors, including lung cancer, malignant melanoma and hepatocellular cancer (Ye et al. 2018; Wang et al. 2019; Zhuang et al. 2019). Increased PYCR1 expression correlates with tumor progression and predicts poor prognosis in cancer patients (Ding et al. 2017; Weijin et al. 2019). Moreover, PYCR1 inhibition has been shown to inhibit tumor formation in vitro and in vivo (Zeng et al. 2017; Yan et al. 2019; Zhuang et al. 2019), which indicates the potential value of PYCR1 as a target for development of anticancer therapy.
The canonical Wnt/β-catenin signaling modulates various fundamental processes that are essential for embryonic development and adult tissue homeostasis (Niehrs 2012). β-catenin protein is a core component of the Wnt/β-catenin signal transduction pathway. In response to signal, β-catenin is stabilized and is translocated into the nucleus where it binds members of the TCF/LEF (T cell factor/lymphoid enhancer factor) family of transcription factors to initiate transcription of Wnt/β-catenin target genes (Mosimann et al. 2009). Under basal conditions, β-catenin is a highly unstable protein that undergoes proteasomal degradation that is mediated by destruction complex. Glycogen synthase kinase-3β (GSK-3β) is a core kinase component of the destruction complex, which mediates the phosphorylation of β-catenin required for proteasomal degradation (Nakamura et al. 1998). The activation status of GSK-3β depends on the phosphorylation status of its Ser9 residue. Phosphorylation of GSK-3β by Akt results in the inactivation of GSK-3β (Cross et al. 1995). Therefore, the Akt/GSK-3β axis exerts a key role in modulating the activation of Wnt/β-catenin signaling. Aberrant activation of Wnt/β-catenin signaling occurs frequently in various cancers, including bladder cancer (Duchartre et al. 2016; Garg and Maurya 2019). Enhancing our understanding of the molecular mechanisms involved in regulating Wnt/β-catenin signaling will enhance our understanding of the pathogenesis of bladder cancer.
Accumulating evidence indicates that PYCR1 dysregulation is related to the development and progression of various cancer types (Ye et al. 2018; Wang et al. 2019; Zhuang et al. 2019). Yet, the relevance of PYCR1 in bladder cancer has not yet been well studied. The study was performed to enhance our understanding of the potential relevance of PYCR1 in bladder cancer. Our data reveal that PYCR1 expression is increased in bladder cancer tissues, and elevated expression of PYCR1 in bladder cancer patients predicted shortened survival rates. Functional experiments demonstrated that PYCR1 knockdown retarded proliferation and invasion of bladder cancer cells, while PYCR1 overexpression had opposite effects. Moreover, PYCR1 knockdown restricted the activation of Wnt/β-catenin signaling via inhibition of Akt. Overexpression of β-catenin partially reversed PYCR1 knockdown-mediated inhibition of tumor formation. Notably, PYCR1 knockdown significantly impeded bladder cancer cell tumorigenesis in vivo. Taken together, results of this study demonstrate that PYCR1 is highly expressed in bladder cancer and exerts a potential tumor-promoting function via modulation of Akt/Wnt/β-catenin signaling. Our study highlights the potential role of PYCR1 in bladder cancer progression and suggests that PYCR1 represents an attractive potential target for treatment of bladder cancer.
Materials and methods
Bioinformatics analysis
The online database Gene Expression Profiling Interactive Analysis 2 (GEPIA2) (http://gepia2.cancer-pku.cn/#index) was applied to analyze the expression levels of PYCR1 in bladder cancer tissue (n = 404) and normal tissues (n = 28). The association between PYCR1 level and overall survival of bladder cancer was also assessed by GEPIA2.
Bladder cancer specimens
Bladder cancer tissues and adjacent matched non-cancerous tissues were obtained from bladder cancer patients who were diagnosed with primary bladder cancer and underwent transurethral resection or partial cystectomy at Shaanxi Provincial People’s Hospital. Tissue specimens were pathologically confirmed and preserved in liquid nitrogen at −80 °C. All patients provided written informed consent with regard to tissue donation for the purpose of this study. Research was carried out with the approval of Ethics Committee of Shaanxi Provincial People’s Hospital and was conducted in accordance with the principles of the Helsinki Declaration.
Bladder cancer cell lines
Bladder cancer cell lines including T24, 5637, UM-UC-3 and SW780, and a normal bladder epithelial cell line, SV-HUC-1, were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultivated in accordance with manufacturer’s culture methods and grown under a humidified atmosphere of 5% CO2 at 37 °C.
Real-time quantitative PCR (RT-qPCR)
To quantify gene expression, cells or tissue samples were lysed and homogenized in TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) to isolate and purify high-quality total RNA in accordance with the protocol provided. Total RNA provided template for reverse transcription using the PrimeScript RT reagent Kit (TaKaRa Biomedical Technology, Beijing, China) and RT-qPCR to assess gene expression was performed using TB Green Fast qPCR Mix (TaKaRa Biomedical Technology). All data analyses were performed using the 2ΔΔCt method, and results were normalized to GAPDH expression levels to determine the expression of target genes. The primer sequences for RT-qPCR were as follows: PYCR1, sense: 5’-TGGCTGCCCACAAGATAATGG-3′ and antisense: 5’-CGTGACGGCATCAATCAGGT-3′ and GAPDH, sense: 5’-GAAGGTGAAGGTCGGAGTC-3′ and antisense; 5’-GAAGATGGTGATGGGATTTC-3′.
Western blot
Cells or tissue samples were lysed and homogenized in RIPA protein extraction reagent (Beyotime, Shanghai, China) to extract total proteins. Protein separation was performed via sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electro-transfer was carried out to transfer separated proteins from sodium dodecyl sulfate-polyacrylamide gels to polyvinylidene difluoride (PVDF) membranes. Before immunoblotting with primary antibodies, PVDF membranes were immersed in blocking buffer. To visualize protein bands, membranes were treated with horseradish peroxidase-conjugated secondary antibodies (1:10000; Sanying, Wuhan, China) and ECL reagents. Primary antibodies included anti-PYCR1 (1:1000; Abcam, Cambridge, UK), anti-Akt (1:1000; Cell Signaling Technology, Danvers, MA, USA), anti-phospho-Akt (Ser473) (1:2000; Cell Signaling Technology), anti-phospho-GSK-3β (Ser9) (1:1000; Cell Signaling Technology), anti-active β-catenin (1:1000; Cell Signaling Technology), and anti-GAPDH (1:5000; Abcam) antibodies.
Plasmid construction and cell transfection
PYCR1 shRNA sequences (5’-CACCACCAUCCAUGCCUUGCAUGCGAACAUGCAAGGCAUGGAUGG-3′) were inserted into the pLKO.1 plasmid to a generate PYCR1 shRNA plasmid. Scrambled shRNA sequences (5’-CACCACCACCCGUUUCCGAUAUGCGAACAUAUCGGAAACGGGUGGU-3′) were utilized as control. Coding sequences of PYCR1 were subcloned into a pcDNA3.1 plasmid to construct a PYCR1 expression plasmid. Constructed expression or plasmids were transfected into bladder cancer cell lines using Invitrogen Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) in compliance with the manufacturer’s specifications.
Detection of cell proliferation
A Cell Counting Kit-8 (CCK-8) assay was performed to detect cell proliferative rate. Briefly, bladder cancer cells were seeded into 96-well culture plates and cultivated overnight to allow cells to adhere to plates prior to transfection. CCK-8 reagent (Beyotime, Shanghai, China) added to cells was utilized to monitor cell proliferation. The optical density of cell cultures was measured spectrophotometrically at 450 nm using an enzyme-linked immunosorbent assay plate reader (Bio-Rad, Hercules, CA, USA). An EdU assay was performed to detect cell proliferation using an EdU Cell Proliferation Kit (Beyotime) as per the manufacturer’s instructions.
Measurement of cell apoptosis
Annexin V-FITC/PI assay was performed to detect cell apoptotic rate. In brief, bladder cancer cells were trypsinized, washed and counted at the time of detection. Then, 1 × 105 cells were collected and suspended into Annexin V-FITC/PI binding buffer, followed by adding of 5 μl Annexin V-FITC and 10 μl PI reagents. Cells were cultivated for 15 min in the dark before detection. Cell apoptosis was assessed by a flow cytometer.
Assessment of cell invasion
A Transwell invasion assay was performed to assess cell invasion. Transfected bladder cancer cells were trypsinized, resuspended and plated within the top chamber, which was pre-coated with Matrigel. In the top chamber, serum-free medium was added, while 10% serum-containing medium was added to the lower chamber, which served as a chemoattractant for cell invasion. After a 24-h incubation at 37 °C, cells of the upper chamber that had not invaded lower surfaces were cleaned using a cotton swab and the filters were individually fixed and stained for visualizing cells that had invaded lower wells. The number of cells that had invaded were counted using a microscope.
Measurement of colony formation
Recombinant lentivirus expressing PYCR1 shRNA (LV-PYCR1 shRNA) or scrambled shRNA (LV-Scrambled shRNA) were purchased from GenePharma (Shanghai, China). Bladder cancer cells stably infected were LV-PYCR1 shRNA or LV-Scrambled shRNA were trypsinized to obtain single cell suspensions. A total of 1000 cells per well were seeded on a six-well plate and cultivated at 37 °C until small colonies were visible. Afterward, visible colonies were washed, fixed and stained. The colonies were counted using a microscope.
Measurement of proline level
Level of proline was measured according to a previously described protocol (Guo et al. 2019). In brief, cells to be detected were cultured in medium without FBS for 24 h. Then, cells were collected and lysed in PBS containing 1% Triton X-100. The supernatants were collected by centrifugation and boiled for 10 min. Thereafter, 200 μl of the supernatants were collected and incubated with 400 μl of 1.25% ninhydrin at 100 °C for 20 min. Afterwards, the absorbance of the reaction mixture at 508 nm was measured via a microplate reader.
TCF/LEF luciferase reporter assay
Wnt/β-catenin signaling activity in bladder cancer cells was monitored using a TCF/LEF Reporter Kit (Bioscience, San Diego, CA, USA). TCF/LEF luciferase reporter vectors, constitutively-expressing Renilla luciferase vectors, and PYCR1 expression vectors or PYCR1 shRNA were cotransfected into bladder cancer cells, followed by incubation for 48 h. Luciferase activities within cells were assessed using the Dual Luciferase (Firefly-Renilla) Assay System.
Subcutaneous xenograft experiment
Female BALB/c nude mice provided by Laboratory Animal Centre of Xi’an Jiaotong University Health Science Center (Xi’an, China) were utilized for xenograft experiments with the approval of the Ethics Committee of Shaanxi Provincial People’s Hospital. Xenograft experiments were carried out according to a previously described method (Du et al. 2020). UM-UC-3-pLKO.1/PYCR1 shRNA cells stably expressing PYCR1 shRNA were selected using puromycin. UM-UC-3 cells (1 × 107) were inoculated subcutaneously into the left sides of the flank region of the nude mice. When tumors were developed, the width and length of tumors were measured using the calipers. Tumor volume was computed according to the following equation: width2 × length × 0.5.
Statistical analysis
All experimental data were expressed as mean ± standard deviation. GraphPad Prism 8 (Software Inc., San Diego, CA, USA) was utilized to perform statistical analyses. Comparisons were evaluated using a Student’s t test or one-way analysis of variance followed by Tukey’s post hoc test. Differences in which p < 0.05 were deemed statistically significant.
Results
PYCR1 is upregulated in bladder cancer tissue
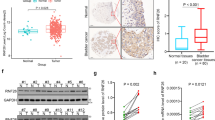
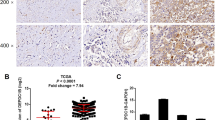
To evaluate the involvement of PYCR1 in bladder cancer, we first examined the expression pattern of PYCR1 in bladder cancer tissue by analyzing data publicly available within the, Gene Expression Profiling Interactive Analysis 2 (GEPIA2) database. Interestingly, we found that PYCR1 expression was markedly elevated in bladder cancer compared with normal tissues (Fig. 1a and b). Survival analysis revealed that bladder cancer patients with high levels of PYCR1 had shorter survival rates than those with low levels of PYCR1 (Fig. 1c). To verify that high levels of PYCR1 expression occur in bladder cancer, we measured levels of PYCR1 mRNA and protein expression in clinical specimens. RT-qPCR analysis showed that PYCR1 mRNA expression was markedly elevated in bladder cancer tissues (Fig. 1d). Correspondingly, Western blot confirmed that PYCR1 protein expression levels were significantly upregulated in bladder cancer tissues (Fig. 1e and f). In summary, these data indicate that increased PYCR1 expression occurs in bladder cancer.
PYCR1 was highly expressed in bladder cancer. a, b Analysis of PYCR1 expression in bladder cancer was performed using data from GEPIA2 (*p < 0.05). N denoted normal tissue and T denoted tumor tissue. c Survival analysis of bladder cancer patients in relation to PYCR1 expression status using data from GEPIA2 (HR denoted Hazard Rate). d Relative PYCR1 mRNA expression levels in clinical bladder cancer specimens were determined via RT-qPCR (N = 6, ***p < 0.001). e, f Protein PYCR1 expression levels in clinical bladder cancer specimens were determined via Western blot (N = 6, ***p < 0.001). Abbreviations: N, normal tissue; T, tumor tissue
Silencing of PYCR1 had tumor-inhibition effect in bladder cancer cells
To explore the precise role of PYCR1 in the regulation of cellular functioning in bladder cancer cells, we performed a series of functional assays. In the assays, we assessed the role of PYCR1 using bladder cancer cell lines. Both PYCR1 mRNA and protein expression levels have been previously shown to be upregulated in multiple bladder cancer cell lines (Fig. 2a–c). Notably, silencing of PYCR1 decreased the level of proline in bladder cancer cells (Supplementary Fig. 1A). Transfection of PYCR1 shRNA expression vectors into T24 and UM-UC-3 cells resulted significantly decreased PYCR1 expression (Fig. 2d–f). Silencing of PYCR1 markedly reduced the proliferative rate of T24 and UM-UC-3 cells (Fig. 2g), as detected by CCK-8 assay. Moreover, the suppressive effect of PYCR1 silencing on the proliferation of T24 and UM-UC-3 cells was confirmed by EdU assay (Fig. 2h and i). Further, PYCR1 silencing enhanced the apoptosis of T24 and UM-UC-3 cells (Fig. 2j and k). PYCR1 silencing decreased the invasive potential of T24 and UM-UC-3 cells (Fig. 2l and m). Additionally, T24 and UM-UC-3 cells stably expressing PYCR1 had reduced capability to form colonies (Supplementary Fig. 2).These results indicate that PYCR1 silencing has antitumor effects in bladder cancer cells.
Silencing of PYCR1 suppressed the proliferation and invasion in bladder cancer cells. a Relative levels of PYCR1 mRNA expression in T24, 5637, UM-UC-3 and SW780 cells were examined via RT-qPCR (N = 3, *p < 0.05 and **p < 0.01 vs. SV-HUC-1). The normal bladder epithelial cell line, SV-HUC-1, was utilized as control. b, c PYCR1 protein expression levels in bladder cancer cells and normal ladder epithelial cells were determined via Western blot (N = 3, *p < 0.05 and **p < 0.01 vs. SV-HUC-1). T24 and UM-UC-3 cells were transfected with pLKO.1 vectors expressing scrambled shRNA or PYCR1 shRNA for 48 h, and d PYCR1 mRNA expression was examined via RT-qPCR (N = 3, ***p < 0.001), and e, f PYCR1 protein expression was assessed via Western blot (N = 3, ***p < 0.001). The effect of PYCR1 silencing on cell proliferation was evaluated using g CCK-8 (N = 5, **p < 0.01) and h, i EdU (N = 3, **p < 0.01) assays. j, k Th effect of PYCR1 silencing on cell apoptosis was assessed via Annexin V-FITC/PI apoptosis assay (N = 3, **p < 0.01). (L, M) The effect of PYCR1 silencing on cell invasion was detected using Transwell invasion assay (N = 3, **p < 0.01)
Upregulation of PYCR1 accelerated the proliferation and invasion of bladder cancer
To validate the role of PYCR1 in bladder cancer, we further assessed the effect of PYCR1 overexpression on bladder cancer cell proliferation and invasion. Transfection of PYCR1 expression vectors caused a marked increase in PYCR1 expression in bladder cancer cells (Fig. 3a and b). Strikingly, overexpression of PYCR1 increased the level of proline in bladder cancer cells (Supplementary Fig. 1B). Upregulation of PYCR1 markedly promoted the proliferation of bladder cancer cells (Fig. 3c-e), but decreased the apoptosis of bladder cancer cells (Fig. 3f and g). Additionally, the invasive potential of bladder cancer cells was also enhanced by overexpression of PYCR1 (Fig. 3h and i). Therefore, these findings confirm that PYCR1 exerts a tumor-promotion function in bladder cancer.
PYCR1 overexpression accelerated bladder cancer cell proliferation and invasion. T24 and UM-UC-3 cells were transfected with either PYCR1 expression or empty vectors (EV) for 48 h, and a, b PYCR1 protein expression was assessed via Western blot (N = 3, **p < 0.01). The effect of PYCR1 overexpression on cell proliferation was evaluated using c CCK-8 (N = 5, **p < 0.01) and d, e EdU (N = 3, **p < 0.01) assays. f, g The effect ofPYCR1 overexpression on cell apoptosis was assessed via Annexin V-FITC/PI apoptosis assay (N = 3, **p < 0.01). h, i The effect of PYCR1 overexpression on cell invasion was determine using a Transwell invasion assay (N = 3, **p < 0.01)
PYCR1 modulates the activation of Akt and Wnt/β-catenin signaling
To illustrate the potential molecular mechanism that underlies PYCR1-mediated effects, we evaluated the influence of PYCR1 on Akt signaling, a key signaling pathway that controls the malignant progression of bladder cancer. Results revealed that PYCR1 knockdown significantly decreased levels of Akt phosphorylation (Fig. 4a). Moreover, PYCR1 knockdown reduced the GSK-3β phosphorylation, and downregulated levels of active β-catenin accumulation (Fig. 4a). Moreover, PYCR1 knockdown markedly inhibited the activation of Wnt/β-catenin signaling (Fig. 4b). In contrast, PYCR1 overexpression promoted the activation of Akt and Wnt/β-catenin signaling (Fig. 4c and d). Collectively, our data suggest that PYCR1 is involved in the modulation of Akt and Wnt/β-catenin signaling.
PYCR1 modulates the activation of Akt and Wnt/β-catenin signaling. a The effect of PYCR1 knockdown on levels of phospho-Akt, phospho-GSK-3β, and active β-catenin was determined via Western blot. b The effect of PYCR1 knockdown on Wnt/β-catenin signaling was monitored using a TCF/LEF luciferase reporter assay (N = 5, **p < 0.01). c The effect of PYCR1 overexpression on levels of phospho-Akt, phospho-GSK-3β, and active β-catenin were determined via Western blot. d The effect of PYCR1 overexpression on Wnt/β-catenin signaling was monitored using a TCF/LEF luciferase reporter assay (N = 5, **p < 0.01)
Inhibition of Akt abolished the promotion effect of PYCR1 overexpression on the activation of Wnt/β-catenin signaling
To validate whether PYCR1 modulates Wnt/β-catenin signaling via Akt, we assessed the effect of inhibiting Akt on PYCR1-mediated activation of Wnt/β-catenin signaling. We demonstrated that inhibition of Akt with GSK690693 markedly decreased expression of active β-catenin (Fig. 5a), and downregulated the activation of Wnt/β-catenin signaling (Fig. 5b). Notably, Akt inhibition blocked PYCR1 overexpression-mediated activation of Wnt/β-catenin signaling (Fig. 5a and b). Further, PYCR1 overexpression-mediated oncogenic effects on bladder cancer cell proliferation (Fig. 5c-e) and invasion (Fig. 5f and g) were markedly reversed by Akt inhibition. In summary, these data indicate that PYCR1 promotes the activation of Wnt/β-catenin signaling via modulation of Akt.
PYCR1 enhances activation of Wnt/β-catenin signaling via modulation of Akt. T24 and UM-UC-3 cells were transfected with PYCR1 expression vector or empty vector (EV) and incubated for 48 h with or without 1 μM of GSK690693. a Levels of active β-catenin were determined via Western blot. b Activation of Wnt/β-catenin signaling was monitored using a TCF/LEF luciferase reporter assay (N = 5, **p < 0.01). Cell proliferation was assessed using (c) CCK-8 (N = 5, **p < 0.01) and d, e EdU (N = 3, **p < 0.01) assays. f, g Cell invasion was evaluated via Transwell invasion assay (N = 3, **p < 0.01)
Reactivation of Wnt/β-catenin reversed PYCR1 knockdown-mediated tumor-inhibition
To evaluate whether Wnt/β-catenin signaling contributes to PYCR1-mediated effects on bladder cancer progression, we determined the effect of Wnt/β-catenin reactivation on PYCR1 knockdown-mediated tumor suppression. Transfection with β-catenin expression vectors significantly reactivated Wnt/β-catenin signaling in PYCR1 shRNA-transfected bladder cancer cells (Fig. 6a and b). As expected, the suppressive effect of PYCR1 knockdown on bladder cancer cell proliferation (Fig. 6c-e) and invasion (Fig. 6f and g) was partially reversed by β-catenin overexpression. Collectively, these data confirm that Wnt/β-catenin signaling contributes to the PYCR1-mediated regulation of bladder cancer progression.
Reactivation of Wnt/β-catenin signaling reversed PYCR1 knockdown-mediated tumor-inhibition. T24 and UM-UC-3 cells were cotransfected with PYCR1 shRNA and β-catenin expression vectors for 48 h, and a levels of β-catenin proteins were examined via Western blot. b Activation of Wnt/β-catenin signaling was evaluated via TCF/LEF luciferase reporter assay (N = 5, **p < 0.01). Cell proliferation was measured using c CCK-8 (N = 5, **p < 0.01) and d, e EdU (N = 3, **p < 0.01) assays. f, g Cell invasion was determined via Transwell invasion assay (N = 3, **p < 0.01)
Knockdown of PYCR1 impeded the tumorigenesis of bladder cancer in vivo
To evaluate whether inhibition of PYCR1 expression inhibited tumor formation in vivo, we carried out a xenograft tumor experiment in nude mice. Inoculation of UM-UC-3 cells in which PYCR1 was knocked out reduced levels of tumor formation (Fig. 7a and b). Moreover, PYCR1 knockdown decreased levels of Akt and GSK-3β phosphorylation, and downregulated protein expression levels of active β-catenin in tumor tissues (Fig. 7c). These data verify that PYCR1 has the potential to be utilized a therapeutic target for the treatment of bladder cancer.
Knockdown of PYCR1 impeded bladder cancer tumorigenesis in vivo. The effect of PYCR1 knockdown on a a tumor growth curve (N = 5, *p < 0.05 and **p < 0.01) and b tumor weight (N = 5, **p < 0.01) was investigated using a xenograft tumor experiment in nude mice. c The effect of PYCR1 knockdown on phosphor-Akt, phosphor-GSK-3βand active β-catenin expression in tumor tissues was detected via Western blot. d A graphical model of PYCR1-medieated Akt/GSK-3β/Wnt/β-catenin signaling in bladder cancer
Discussion
Our study has demonstrated that PYCR1 plays a vital role in bladder cancer progression. Our results revealed that PYCR1 expression was greatly increased in bladder cancer compared with normal tissues. Moreover, bladder cancer patients with high PYCR1 expression had significantly lower survival rates than those expressing lower levels of PYCR1. Functional studies revealed that knockdown of PYCR1 decreased levels of bladder cancer cell proliferation and invasion, while PYCR1 overexpression promoted tumor formation. Notably, we were able to show that promotion of tumor formation by PYCR1 depended on its capacity to regulate the Akt/GSK-3β/Wnt/β-catenin signaling axis (Fig. 7d). Our study suggests that PYCR1acts as a critical modulator of Wnt/β-catenin signaling and likely regulates the progression of bladder cancer.
PYCR1 is aberrantly expressed in various cancers. Both PYCR1 mRNA and protein expression are significantly elevated in breast cancer, and levels of PYCR1 are correlated with tumor size and grade (Ding et al. 2017). Moreover, elevated PYCR1 expression levels are commonly detected lung tumors, malignant melanoma and colorectal cancer (Cai et al. 2018; Ye et al. 2018; Yan et al. 2019; Zhuang et al. 2019). Notably, enhanced expression of PYCR1 predicts low survival rates in cancer patients, suggesting that PYCR1 may be of value as a prognostic biomarker (Ding et al. 2017; Cai et al. 2018; Ye et al. 2018; Gao et al. 2020). Interestingly, increased PYCR1 expression has also been detected in several urinary cancers including prostate cancer and renal cancer (Zeng et al. 2017; Wang and Liu 2019; Weijin et al. 2019). However, little is known about the role of PYCR1 in bladder cancer. In this study, for the first time, we reported that PYCR1 expression was upregulated in bladder cancer, by analyzing sequencing data using a publicly available database. In addition, we verified that PYCR1 expression was also elevated in clinical specimens of bladder cancer via RT-qPCR and Western blot analysis. Notably, analysis of survival data revealed that elevated PYCR1 expression predicted significantly decreased survival rates in bladder cancer patients. Thus, our study confirms that PYCR1 is highly expressed in bladder cancer and is an attractive potential biomarker of bladder cancer prognosis.
PYCR1 has been reported to plays a key role in regulating malignant behaviors of cancer cells. PYCR1 accelerates non-small cell lung cancer progression by promoting cellular proliferation and inhibiting apoptosis (Cai et al. 2018; Wang et al. 2019). Knockdown of PYCR1 caused a marked reduction in proliferation and invasion in various cancer cell types (Cai et al. 2018; Ye et al. 2018; Yan et al. 2019; Zhuang et al. 2019). In line with these findings, our data demonstrated that PYCR1 knockdown markedly suppressed the proliferation and invasion of bladder cancer cells. Moreover, in vivo experiments showed that PYCR1 knockdown restricted the tumor formation and growth of bladder cancer cells, confirming PYCR1 may serve as an attractive anticancer target. Further, PYCR1 overexpression accelerated bladder cancer cell proliferation and invasion, suggesting that increased expression of PYCR1 may promote the progression of bladder cancer.
Increasing numbers of studies have reported that PYCR1 facilitates tumor progression by affecting various signaling pathways (Yan et al. 2019; Zhuang et al. 2019; Gao et al. 2020). Interestingly, PYCR1 has been reported to be a key regulator of oncogenic Akt signaling. In malignant melanoma, PYCR1 knockdown decreased levels of Akt phosphorylation and expression of downstream targets of Akt signaling (Ye et al. 2018). PYCR1 inhibition also suppressed Akt activation in papillary renal cell carcinoma (Wang and Liu 2019). Considering that Akt signaling contributes to the development and progression of bladder cancer (Sathe and Nawroth 2018), we investigated the regulatory effect of PYCR1 on Akt activation in bladder cancer cells. Intriguingly, we found that knockdown of PYCR1 markedly decreased levels of Akt phosphorylation, while PYCR1 overexpression enhanced activation of Akt. These findings indicate that PYCR1 regulates Akt activation in bladder cancer. Moreover, we found that PYCR1 modulated Wnt/β-catenin signaling via Akt. Reactivation of Wnt/β-catenin signaling markedly reversed PYCR1 knockdown-mediated inhibition of tumor formation. Taken together, these data indicate that PYCR1-mediated Akt/Wnt/β-catenin signaling may represent a new molecular mechanism underlying bladder cancer progression. However, we found that in β-catenin-overexpressing cells, PYCR1 knockdown still have effects on the proliferation and invasion of bladder cancer cells, indicating that there are also other targets mediated by PYCR1 contributing to bladder cancer progression.
In summary, our results indicate that PYCR1 expression is elevated in bladder cancer and may serve as a potential biomarker of bladder cancer prognosis. Our study indicates that overexpression of PYCR1 promotes bladder cancer cell proliferation and invasion through modulation of Akt/Wnt/β-catenin signaling. Therefore, PYCR1-mediated Akt/Wnt/β-catenin signaling may play a role in bladder cancer progression and PYCR1 is an attractive potential therapeutic target for the treatment of bladder cancer.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adams E, Frank L (1980) Metabolism of proline and the hydroxyprolines. Annu Rev Biochem 49:1005–1061
Berdik C (2017) Unlocking bladder cancer. Nature 551:S34–S35
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Cai F, Miao Y, Liu C, Wu T, Shen S, Su X, Shi Y (2018) Pyrroline-5-carboxylate reductase 1 promotes proliferation and inhibits apoptosis in non-small cell lung cancer. Oncol Lett 15:731–740
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789
De Ingeniis J, Ratnikov B, Richardson AD, Scott DA, Aza-Blanc P, De SK, Kazanov M, Pellecchia M, Ronai Z, Osterman AL, Smith JW (2012) Functional specialization in proline biosynthesis of melanoma. PLoS One 7:e45190
Ding J, Kuo ML, Su L, Xue L, Luh F, Zhang H, Wang J, Lin TG, Zhang K, Chu P, Zheng S, Liu X, Yen Y (2017) Human mitochondrial pyrroline-5-carboxylate reductase 1 promotes invasiveness and impacts survival in breast cancers. Carcinogenesis 38:519–531
Du S, Zhang P, Ren W, Yang F, Du C (2020) Circ-ZNF609 accelerates the radioresistance of prostate cancer cells by promoting the glycolytic metabolism through miR-501-3p/HK2 axis. Cancer Manag Res 12:7487–7499
Duchartre Y, Kim YM, Kahn M (2016) The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol 99:141–149
Gao Y, Luo L, Xie Y, Zhao Y, Yao J, Liu X (2020) PYCR1 knockdown inhibits the proliferation, migration, and invasion by affecting JAK/STAT signaling pathway in lung adenocarcinoma. Mol Carcinog 59:503–511
Garg M, Maurya N (2019) WNT/beta-catenin signaling in urothelial carcinoma of bladder. World J Nephrol 8:83–94
Guo L, Cui C, Zhang K, Wang J, Wang Y, Lu Y, Chen K, Yuan J, Xiao G, Tang B, Sun Y, Wu C (2019) Kindlin-2 links mechano-environment to proline synthesis and tumor growth. Nat Commun 10:845
Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, Guo CC, Lotan Y, Kassouf W (2016) Bladder cancer. Lancet 388:2796–2810
McConkey DJ, Lee S, Choi W, Tran M, Majewski T, Lee S, Siefker-Radtke A, Dinney C, Czerniak B (2010) Molecular genetics of bladder cancer: emerging mechanisms of tumor initiation and progression. Urol Oncol 28:429–440
Mosimann C, Hausmann G, Basler K (2009) Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol 10:276–286
Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T (1998) Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells 3:395–403
Niehrs C (2012) The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 13:767–779
Pang C, Guan Y, Li H, Chen W, Zhu G (2016) Urologic cancer in China. Jpn J Clin Oncol 46:497–501
Reversade B, Escande-Beillard N, Dimopoulou A, Fischer B, Chng SC, Li Y, Shboul M, Tham PY, Kayserili H, Al-Gazali L, Shahwan M, Brancati F, Lee H, O'Connor BD, Schmidt-von Kegler M, Merriman B, Nelson SF, Masri A, Alkazaleh F, Guerra D, Ferrari P, Nanda A, Rajab A, Markie D, Gray M, Nelson J, Grix A, Sommer A, Savarirayan R, Janecke AR, Steichen E, Sillence D, Hausser I, Budde B, Nurnberg G, Nurnberg P, Seemann P, Kunkel D, Zambruno G, Dallapiccola B, Schuelke M, Robertson S, Hamamy H, Wollnik B, Van Maldergem L, Mundlos S, Kornak U (2009) Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet 41:1016–1021
Sathe A, Nawroth R (2018) Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol 1655:335–350
Wang D, Wang L, Zhang Y, Yan Z, Liu L, Chen G (2019) PYCR1 promotes the progression of non-small-cell lung cancer under the negative regulation of miR-488. Biomed Pharmacother 111:588–595
Wang QL, Liu L (2019) PYCR1 is associated with papillary renal cell carcinoma progression. Open Med (Wars) 14:586–592
Weijin F, Zhibin X, Shengfeng Z, Xiaoli Y, Qijian D, Jiayi L, Qiumei L, Yilong C, Hua M, Deyun L, Jiwen C (2019) The clinical significance of PYCR1 expression in renal cell carcinoma. Medicine 98:e16384
Witjes JA, Comperat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A (2014) EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 65:778–792
Yan K, Xu X, Wu T, Li J, Cao G, Li Y, Ji Z (2019) Knockdown of PYCR1 inhibits proliferation, drug resistance and EMT in colorectal cancer cells by regulating STAT3-mediated p38 MAPK and NF-kappaB signalling pathway. Biochem Biophys Res Commun 520:486–491
Yasuda T, Kaji Y, Agatsuma T, Niki T, Arisawa M, Shuto S, Ariga H, Iguchi-Ariga SM (2013) DJ-1 cooperates with PYCR1 in cell protection against oxidative stress. Biochem Biophys Res Commun 436:289–294
Ye Y, Wu Y, Wang J (2018) Pyrroline-5-carboxylate reductase 1 promotes cell proliferation via inhibiting apoptosis in human malignant melanoma. Cancer Manag Res 10:6399–6407
Zeng T, Zhu L, Liao M, Zhuo W, Yang S, Wu W, Wang D (2017) Knockdown of PYCR1 inhibits cell proliferation and colony formation via cell cycle arrest and apoptosis in prostate cancer. Med Oncol 34:27
Zhuang J, Song Y, Ye Y, He S, Ma X, Zhang M, Ni J, Wang J, Xia W (2019) PYCR1 interference inhibits cell growth and survival via c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathway in hepatocellular cancer. J Transl Med 17:343
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Shuangkuan Du designed the study, performed the experiments and drafted the manuscript. Yongjie Sui designed the study and revised the manuscript. Wei Ren performed the experiments. Jiancheng Zhou interpreted the data. Chun Du interpreted the data. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Research was carried out with the approval of Ethics Committee of Shaanxi Provincial People’s Hospital and was conducted in accordance with the principles of the Helsinki Declaration. Animal experiments were performed with the approval of the Ethics Committee of Shaanxi Provincial People’s Hospital.
Consent to participate
All patients provided written informed consent with regard to tissue donation for the purpose of this study.
Consent for publication
All authors have approved for the publication of this manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 497 kb)
Rights and permissions
About this article
Cite this article
Du, S., Sui, Y., Ren, W. et al. PYCR1 promotes bladder cancer by affecting the Akt/Wnt/β-catenin signaling. J Bioenerg Biomembr 53, 247–258 (2021). https://doi.org/10.1007/s10863-021-09887-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-021-09887-3