Abstract
Non-small cell lung cancer (NSCLC) constitutes nearly 85% of all cases of lung cancer. Drug resistance, dose-limiting toxicity, and metastasis in NSCLC eventually reduce the efficacy of chemotherapeutics. In this study, we have shown that the methanol-ethyl acetate partitioned fraction from Magnolia grandiflora L. seeds (MEM) exhibit potential anti-cancer activities against NSCLC H1975 cells in vivo and in vitro. MEM significantly inhibited the proliferation of H1975 cells in a concentration- and time-dependent manner. Further, MEM exhibited potent anti-tumor efficacy and low toxicity in nude mice bearing H1975 tumors. Our study also showed that MEM could induce cellular apoptosis in H1975 cells by down-regulating the protein expression levels of Akt and p-Akt-473, and by increasing the ratio of Bax/Bcl-2. Also, MEM significantly inhibited metastasis-related cell invasion and migration of H1975 cells, which associated with the down-regulation of HIF-1α, MMP-2, and MMP-9 protein expression levels. Thus, our data shows that MEM may be an effective fraction of M. grandiflora in NSCLC treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the most common causes of cancer and life ends in death (Bray et al. 2012). Non-small cell lung cancer (NSCLC) accounts for 85% cases of lung cancer cases and has been treated by drugs targeting receptor tyrosine kinases, mainly the epidermal growth factor receptor (EGFR) family (Jemal et al. 2011; Lynch et al. 2006). In the past several years, EGFR-tyrosine kinase inhibitor (TKI), gefitinib (Iressa), and erlotinib (Tarceva), have been the eutherapeutics for patients with NSCLC (Mitsudomi et al. 2010). However, acquired drug-resistance and side effects universally developed in many patients that responded to EGFR-TKIs (Jackman et al. 2010; Lu et al. 2018). Therefore, research and development efforts for discovering highly effective and low toxicity anti-NSCLC drugs have become indispensable for the treatment of NSCLC.
Traditional Chinese medicine (TCM) and natural medicine can serve as one of the most important sources for discovering new drugs with several advantages, such as unique biological activities, multiple targets, low toxicity, and the ability to circumvent drug-resistance (Stone 2008). In our search for novel biologically active agents from TCM and natural medicine, the methanol-ethyl acetate partitioned fraction from Magnolia grandiflora L. seeds (MEM) exhibited potential anti-tumor efficacy and low toxicity in H1975 cells. As a traditional natural medicine, extracts of M. grandiflora L. that contain alkaloids, flavonoids, lignans, phenolic alcohols, terpenes, and sesquiterpene lactones have been widely used for the treatment of fever, diarrhea, abdominal diseases, and rheumatic arthritis (Feltenstein et al. 2004; Li et al. 2015; Schühly et al. 2001). However, the role of MEM in anti-cancer mechanisms of H1975 cells remains unknown. In this study, we have reported the effects of MEM on proliferation, apoptosis, invasion, and migration in NSCLC; and the preliminary mechanisms involved.
Materials and methods
General experimental procedures
RPMI-1640 and 0.25% trypsin were purchased from Hyclone (UT, USA). Fetal bovine serum (FBS) was purchased from Sijiqing (Hangzhou, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H- tetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 17-DMAG was purchased from Selleckchem (Houston, MO, USA). The following antibodies were used: anti-Akt and anti-p-Akt-473 (CST, USA); anti-Bcl-2, anti-Bax and anti-β-actin (Proteintech, USA); anti-HIF-1α, anti-MMP-2 and anti- MMP-9 (Abcam, USA).
Plant material and extraction
M. grandiflora L. seeds were collected in October 2012 from a farm located in Bengbu city, and the authenticity of the plant was confirmed by Prof. Huizi Lv (School of Pharmacy, Yanbian University). A total of 5 kg of well-dried seeds of M. grandiflora L. were macerated in methanol (20 L) twice at room temperature for 3 days. Then the dried methanol extract (303 g) was dissolved with 80% methanol and successively extracted by n-hexane, ethyl acetate and n-butyl alcohol of same volume twice. The filtration of the extracted solutions and evaporation under reduced pressure yielded n-hexane extract (17.7 g), ethyl acetate extract (MEM, 41.2 g), butyl alcohol extract (30.2 g) and water extract (206.8 g). And the extract was determined by HPLC at 254 nm (Fig. S1).
Cell viability assay
The human lung cancer H1975 cells were purchased from Shanghai Cell Bank (China). The cells were maintained in RPMI-1640 with 15% fetal bovine serum (Sijiqing, China) and 1% penicillin/ streptomycin (Gibco, USA) at 37 °C. H1975 cells were seeded in 96-well plate at a density of 8000 cells/ well and were grown overnight, and then treated with various concentrations of extracted samples. The cell viability was evaluated by standard MTT assay procedures. 17-DMAG was used as a positive control.
Colony formation assay
H1975 cells were cultured in 6-well plate at a concentration of 1 × 104 cells/ well overnight. When the cells formed colonies, the medium was replaced with fresh medium containing MEM at low concentrations (2.5, 5, and 10 µg/ml), and the cells were cultured for 96 h. After treatment, cells were washed with PBS, fixed with 4% paraformaldehyde, stained with 2% crystal violet, washed with distilled water, and dried at room temperature, before being quantitatively analyzed.
In vivo anti-tumor experiments
H1975 cells (4 × 106 cells per mouse) were injected subcutaneously into nude mice (5–6 weeks old, purchased from Slac Laboratory, Shanghai, China) to induce tumor formation. When tumor volume reached 50–80 mm3, mice were randomly grouped into vehicle control, MEM, and 17-DMAG groups (3 mice per group). Next, 0.2 ml of physiological saline, MEM (100 mg/kg), and 17-DMAG (10 mg/kg) were intraperitoneally injected every 3 days; tumor volume and body weights were monitored following injection. After treatment for 24 days, the mice were sacrificed and tumor weight, glutamate oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT) levels in the serum were measured. In addition, the tumor, liver, kidney and lung were removed and stored in 4% formalin solution, and then the hematoxylin and eosin (H&E) staining was used for analysis. 17-DMAG was used as a positive control.
Flow cytometry with Annexin V/ PI staining
H1975 cells were seeded in 6-well plate at a density of 3 × 105 cells/well overnight. After cells adhered to the wells, various concentrations of MEM (15, 30, and 60 µg/ml) were added to the cells for 24 h. Cells were harvested, washed with PBS, and centrifuged. To further detect apoptosis, flow cytometry with Annexin V/PI dual staining of the cells was conducted according to the manufacturer’s instructions.
Western blotting
H1975 cells were seeded in 6-well plates at a density of 3 × 105 cells/well and were cultured following treatment with various concentrations of MEM for 24 h. The cells were harvested, homogenized in RIPA lysis buffer at 4 °C; and bicinchoninic acid (BCA) assay kit was used to determine protein concentrations. Equal amounts of proteins were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% skim milk in TBST containing 0.1% Tween-20 and were incubated with primary antibodies for overnight at 4 °C followed by incubation with corresponding secondary antibodies. The protein bands were imaged with gel imaging equipment (Bio-Rad, USA).
Cell invasion assay
Cell invasion assay was performed using a transwell chamber equipped with 8.0 µm pore membrane inserts (Corning, NY) that were coated with 50 µl of matrigel (BD, USA); the traswell chamber was incubated with serum-free media for 1 h at 37 °C. The H1975 cells were seeded in 24-well plates at a density of 1 × 105 cells/ well and were cultured following treatment with various concentrations of MEM for 48 h. The bottom chamber of the transwell was filled with 800 µl of RPMI-1640 supplemented with 15% FBS, and incubated for 48 h. After incubation, the cells were incubated with 4% paraformaldehyde for 15 min and stained with 0.1% crystal violet for 15 min. Once air dried, the stained cells were enumerated under a light microscope at 200 × magnification. The number of invasive cells were counted and analyzed to determine statistically significant differences.
Cell migration assay
The migration assay was performed using a transwell chamber equipped with 8.0 µm pore membrane inserts without matrigel. The cells were seeded in 24-well plates at a density of 5 × 104 per well and were cultured following treatment with various concentrations of MEM for 24 h. The cell migration assay was carried out as mentioned above.
Statistical analysis
All experiments were repeated at least three times and the data are presented as the mean ± SD. SPSS v.16.0 software (SPSS Inc., Chicago, IL, USA) was used for data analysis. Statistical comparisons between groups were carried out using one-way ANOVA followed by Student’s t-test. *P < 0.05 or **P < 0.01 indicates statistical significance.
Results
MEM exhibits anti-proliferative activities in human non-small cell lung cancer H1975 cells
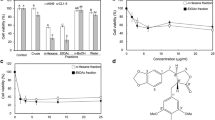
The methanol extract of M. grandiflora L. seeds was partitioned successively using n-hexane, ethyl acetate, and butyl alcohol; and was then analyzed using HPLC at 254 nm (Fig. S1). To understand the anti-cancer activity of different extracts of M. grandiflora in vitro, MTT assay was conducted to determine the cell viability of NSCLC H1975 cells. As shown in Table 1, the preliminary screening revealed that MEM exhibited the best inhibitory activity on the cell viability of H1975 cells. MEM significantly inhibited the viability of H1975 cells in concentration- and time- dependent manner (Fig. 1a). Meanwhile, the colony-formation assay also displayed the anti-proliferative activity of MEM at low concentrations (Fig. 1b and c).
Anti-proliferative activities of MEM in H1975 cells. a Effect of MEM on H1975 cell viability for 24, 48 and 72 h by MTT assays (n = 3). b Effect of MEM on H1975 cells by colony-formation for 96 h. c Quantification of colony forming capability of H1975 cells inhibited by MEM (n = 3). *P<0.05 and ** P<0.01 compared with the control
Anti-tumor efficacy of MEM in H1975 cell xenografts in nude mice
We further investigated the anti-tumor efficacy of MEM in vivo in nude mice bearing H1975 tumors. The tumor-bearing mice were intraperitoneally injected with vehicle, MEM, and 17-DMAG for 24 days. We observed that MEM significantly suppressed tumor growth and reduced the tumor volume to an average of 358.9 mm3 as compared with 1201.5 mm3 in control untreated mice (Fig. 2a and b). Consistent with the tumor volume data, the mice treated with MEM also showed decreased tumor weights by approximately 53.8% (Fig. 2c). However, the body weight increased after treatment with MEM as compared to the control group (Fig. 2d). Next, we examined the levels of GOT and GPT used as biomarkers for the evaluation of hepatotoxicity. As shown in Fig. 2e, MEM treated group had nearly no effect on the GOT and GPT levels as compared with the control group. Hematoxylin and eosin (H&E) staining demonstrated that the liver, kidney and lung had no obvious damage due to treatment with MEM (Fig. 2f). These results suggested that MEM presented potential anti-tumor efficacy and low hepatotoxicity for NSCLC treatment in vivo.
MEM exhibited anti-tumor efficacy in H1975 cell xenograft in nude mice. a Representative images from vehicle, MEM (100 mg/kg) and 17-DMAG (10 mg/kg) treatment group on H1975 cell xenograft in nude mice at the end of the experiment (n = 3). b Tumor volumes of nude mice were estimated. c The solid tumor were removed and weighed at 24 days after inoculation. d Body weights of mice were measured every 3 days. eIn vivo hepatotoxicity evaluation of MEM by GOT and GPT levels of blood serum samples, as described in the “Materials and methods” section. f H&E staining (original magnification 200×) of the tumor, liver, kidney and lung from the mice after vehicle, MEM and 17-DMAG treatment. ** P<0.01 compared with the control
MEM induces apoptosis in human non-small cell lung cancer H1975 cells
Next, we investigated if the effect of MEM on H1975 cell death involved apoptosis by utilizing flow cytometry. As shown in Fig. 3a, the rate of apoptosis in the control cells was 7%, while the rate of apoptosis was 13.1%, 20.7%, and 41.8% for the cell treated with 15, 30, and 60 µg/ml of MEM, respectively. In addition, western blotting analysis demonstrated that MEM gradually down-regulated Akt and p-Akt-473 protein expression levels and increased the Bax/Bcl-2 ratio in a concentration- dependent manner (Fig. 3b).
MEM induced apoptosis in H1975 cells. a Flow cytometry with Annexin V/PI staining was used to measure apoptosis in H1975 cells exposed to various concentration of MEM. b The detection of MEM induced regulation of apoptosis-related proteins (Bcl-2, Bax, Akt and p-Akt-473) by western blotting. Results presented are three independent experiments
Effect of MEM on the invasion and migration of human non-small cell lung cancer H1975 cells
Finally, we examined the invasive ability of H1975 cells treated with MEM using the transwell assay. Our results showed that MEM significantly suppressed the invasion of H1975 cells in a concentration- dependent manner and the number of invading cells was decreased (Fig. 4a and c). Meanwhile, the effect of MEM on cell migration was evaluated by the transwell assay. MEM exhibited a concentration- dependent inhibition of H1975 cells migration and the number of migrating cells was gradually reduced (Fig. 4b and d). In order to reveal the mechanism of how MEM suppressed migration and invasion of H1975 cells, the levels of associated proteins in migration and invasion of H1975 cells was further estimated by Western blotting. The results indicated that HIF-1α, MMP-2, and MMP-9 proteins were gradually down-regulated with increasing concentrations of MEM in H1975 cells (Fig. 4e).
MEM suppressed the invasion and migration of H1975 cells. a The H1975 cells were subjected to the treatment with various concentration of MEM for 48 h, and cell invasion was determined by transwell matrigel assay (original magnification 200×). b The H1975 cells were treated with various concentration of MEM for 24 h, and the ability of cell migration was detected by transwell assay (original magnification 200×). c Quantification of invasion of H1975 cells suppressed by MEM (n = 3). d Quantification of migration of H1975 cells suppressed by MEM (n = 3). e The detection of MEM decreased HIF-1α, MMP-2 and MMP-9 proteins by western blotting. *P<0.05 and ** P<0.01 compared with the control. Data presented are means ± SD of three independent experiments
Discussion
Medicinal plants have increasingly become an attractive source of novel therapeutic agents that may play an essential role in treating cancer. Several studies have been reported on the anti-tumor effects of M. grandiflora in different human cancer cell lines (Li et al. 2015; Marin and Mansilla 2010; Mohamed et al. 2010; Farag and Al-Mahdy 2013). However, the anti-tumor activities and mechanism of M. grandiflora seeds on H1975 cell line are unknown. In this study, we found that MEM exhibited potential anti-tumor effects on H1975 cells in vivo and in vitro, including inhibiting cell viability, inducing apoptosis, and suppressing invasion and migration. Furthermore, previous studies have indicated that active ingredients from TCM can enhance the therapeutic efficacy and decrease the adverse effects in the treatment of lung cancer, which is of significance in improving the quality of life and for increasing the survival time for patients with NSCLC (Xu et al. 2019; Wu et al. 2015).
In this study, systematic solvent partition and MTT assays were used for screening the effective fraction of M. grandiflora seeds. Interestingly, MEM fraction is different from the previously reported active compounds from M. grandiflora, such as bishonokiol A, honikiol, and magnolol (Fig. S1). Generally, the effective fractions of natural medicines have the advantages of having multiple targets, synergistic effects, and low toxicity (Chao et al. 2017). Thus, our research may provide a basis for the screening and discovery of new active compounds and effective fractions from M. grandiflora.
The efficacy of drug depends not only on the binding efficiency with its target, but is also closely related to the overall effect of drugs in vivo, including absorption, distribution, metabolism and excretion (ADME) (Carrara et al. 2017). In vitro experiments showed that MEM had strong anti-proliferative activities against H1975 cell line. The model of nude mice bearing H1975 cells was used to evaluate the anti-tumor effect and hepatotoxicity in order to explore the possibility of clinical application of MEM. Fortunately, the anti-NSCLC activity of MEM in vitro was consistent with the effect in vivo, exhibiting the potential as an effective candidate agent in the treatment of NSCLC.
In the present study, the flow cytometric results showed that the percentage of apoptotic cells was significantly increased after MEM treatment. Hence, the possible molecular mechanism underlying MEM-induced apoptosis of human H1975 cells requires further research. The activation of apoptotic pathway serves an important role in tumor development. Studies have shown that the development and progression of NSCLC are closely associated with the disorders of the PI3K/Akt signaling pathway (Schuurbiers et al. 2009). The PI3K/Akt signaling pathway is an important signal transduction pathway which has been reported to play a critical role in apoptosis (Guo et al. 2015).
In the current study, the association between MEM and the PI3K/Akt pathway was investigated in order to elucidate the apoptotic mechanism. The serine/threonine kinase Akt, also known as protein kinase B, is a major signal transducer of the PI3K/Akt pathway in all cells. A variety of growth factors and signaling proteins can activate Akt by the phosphorylation of Akt at Ser473 and Thr308, phosphorylated Akt plays a pivotal role in the proliferation and apoptosis of tumor cells by regulating its downstream effectors (Manning and Toker 2017; Kumar et al. 2018). Accordingly, phosphorylated Akt is overexpressed in a multitude of human cancers (Hers et al. 2011). The previous studies showed that activated Akt not only can phosphorylate pro-apoptotic proteins in the Bcl-2 family and activate the release of downstream anti-apoptotic protein Bcl-2 to inhibit cell apoptosis, but also directly phosphorylate pro-apoptotic protein Bax to block its pro-apoptotic effect (Manning and Cantley 2007; Yamaguchi and Wang 2001; Zhou et al. 2000). Our findings indicated that MEM may induce apoptosis by suppressing PI3K/Akt signaling pathway.
Lung cancer is one of the most lethal cancers, which presents metastases to other vital organs (Mehlen and Puisieux 2006). Several studies suggested that PI3K/Akt signaling pathway may play a crucial role in proliferation and invasiveness in many types of cancer including lung cancer (Kar et al. 2012; Xie et al. 2017). It has previously been shown that the PI3K/Akt pathway promotes cell invasion and metastasis by increasing the expression of HIF-1α. Importantly, HIF-1α has been reported to be overexpressed in NSCLC (Zhou et al. 2017). Moreover, overexpression of MMP-2 and MMP-9 indicate that HIF-1α triggers the activation of MMPs to promote lung cancer metastasis (Baek et al. 2017; Chang et al. 2017). It has been reported that inhibition of PI3K/Akt signaling significantly reduced the MMP-2 and MMP-9 protein expression levels to suppress cell migration (Cheng et al. 2017). In this study, we found that MEM markedly reduced the expression of HIF-1α, MMP-2, and MMP-9; thereby indicating that the inhibition of H1975 cells invasion and migration by MEM may be related to the inhibition of PI3K/Akt signaling pathway, which in turn lowered HIF-1α, MMP-2, and MMP-9 expression.
In summary, we have demonstrated that anti-tumor effects of MEM on H1975 cells are mediated by PI3K/Akt signaling pathway and its downstream targets, including Bcl-2, HIF-1α, and MMPs. These findings suggest that MEM may be a potential anti-cancer agent for treatment of NSCLC.
References
Baek SH, Ko JH, Lee JH, Kim C, Lee H, Nam D, Lee J, Lee SG, Yang WM, Um JY, Sethi G, Ahn KS (2017) Ginkgolic acid inhibits invasion and migration and TGF-induced EMT of lung cancer cells through PI3K/Akt/mTOR inactivation. J Cell Physiol 232(2):346–354
Bray F, Jemal A, Grey N, Ferlay J, Forman D (2012) Global cancer transitions according to the human development index (2008–2030): a population-based study. Lancet Oncol 13(8):790–801
Carrara L, Lavezzi SM, Borella E, De Nicolao G, Magni P, Poggesi I (2017) Current mathematical models for cancer drug discovery. Expert Opin Drug Discov 12(8):785–799
Chang YC, Chan YC, Chang WM, Lin YF, Yang CJ, Su CY, Huang MS, Wu ATH, Hsiao M (2017) Feedback regulation of ALDOA activates the HIF-1α/MMP9 axis to Q7 promote lung cancer progression. Cancer Lett 403:28–36
Chao J, Dai Y, Verpoorte R, Lam W, Cheng YC, Pao LH, Zhang W, Chen S (2017) Major achievements of evidence-based traditional Chinese medicine in treating major diseases. Biochem Pharmacol 139:94–104
Cheng TC, Din ZH, Su JH, Wu YJ, Liu CI (2017) Sinulariolide suppresses cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 and urokinase through the PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar Drugs 15(8):238
Farag MA, Al-Mahdy DA (2013) Comparative study of the chemical composition and biological activities of Magnolia grandiflora and Magnolia virginiana flower essential oils. Nat Prod Res 27(12):1091–1097
Feltenstein MW, Schühly W, Warnick JE, Fischer NH, Sufka KJ (2004) Anti-inflammatory and anti-hyperalgesic effects of sesquiterpene lactones from Magnolia and Bear’s Foot. Pharmacol Biochem Behav 79(2):299–302
Guo H, German P, Bai S, Barnes S, Guo W, Qi X, Lou H, Liang J, Jonasch E, Mills GB, Ding Z (2015) The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics 42(7):343–353
Hers I, Vincent EE, Tavaré JM (2011) Akt signalling in health and disease. Cell Signal 23(10):1515–1527
Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Jänne PA, Lynch T, Johnson BE, Miller VA (2010) Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 28(2):357–360
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Kar S, Palit S, Ball WB, Das PK (2012) Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis 17(7):735–747
Kumar D, Haldar S, Gorain M, Kumar S, Mulani FA, Yadav AS, Miele L, Thulasiram HV, Kundu GC (2018) Epoxyazadiradione suppresses breast tumor growth through mitochondrial depolarization and caspase-dependent apoptosis by targeting PI3K/Akt pathway. BMC Cancer 18(1):52
Li HM, Zhao SR, Huo Q, Ma T, Liu H, Lee JK, Hong YS, Wu CZ (2015) A new dimeric neolignan from Magnolia grandiflora L. seeds. Arch Pharm Res 38(6):1066–1071
Lu X, Yu L, Zhang Z, Ren X, Smaill JB, Ding K (2018) Targeting EGFR (L858R/T790M) and EGFR (L858R/T790M/ C797S) resistance mutations in NSCLC: current development in medicinal chemistry. Med Res Rev 38:1550–1581
Lynch TJ, Adjei AA, Bunn PA Jr, Eisen TG, Engelman J, Goss GD, Haber DA, Heymach JV, Jänne PA, Johnson BE, Johnson DH, Lilenbaum RC, Meyerson M, Sandler AB, Sequist LV, Settleman J, Wong KK, Hart CS (2006) Summary statement: novel agents in the treatment of lung cancer: advances in epidermal growth factor receptor-targeted agents. Clin Cancer Res 12:4365–4371
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274
Manning BD, Toker A (2017) AKT/PKB Signaling: Navigating the Network. Cell 169(3):381–405
Marin GH, Mansilla E (2010) Apoptosis induced by Magnolia Grandiflora extract in chlorambucil- resistant B-chronic lymphocytic leukemia cells. J Cancer Res Ther 6(4):463–465
Mehlen P, Puisieux A (2006) Metastasis: a question of life or death. Nat Rev Cancer 6(6):449–458
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M, West Japan Oncology Group (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancer Oncol 11(2):121–128
Mohamed SM, Hassan EM, Ibrahim NA (2010) Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Nat Prod Res 24(15):1395–1402
Schühly W, Khan I, Fischer NH (2001) The ethnomedical user of Magnoliaceae from the Southeastern United States as leads in drug discovery. Pharm Biol 39:63–69
Schuurbiers OC, Kaanders JH, van der Heijden HF, Dekhuijzen RP, Oyen WJ, Bussink J (2009) The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J Thorac Oncol 4(6):761–767
Stone R (2008) Biochemistry: Lifting the veilon traditional Chinese medicine. Science 319(5864):709–710
Wu SH, Hsiao YT, Kuo CL, Yu FS, Hsu SC, Wu PP, Chen JC, Hsia TC, Liu HC, Hsu WH, Chung JG (2015) Bufalin inhibits NCI-H460 human lung cancer cell metastasis in vitro by inhibiting MAPKs, MMPs, and NF-κB pathways. Am J Chin Med 43(6):1247–1264
Xie Q, Wen H, Zhang Q, Zhou W, Lin X, Xie D, Liu Y (2017) Inhibiting PI3K-AKt signaling pathway is involved in antitumor effects of ginsenoside Rg3 in lung cancer cell. Biomed Pharmacother 85:16–21
Xu Z, Zhang F, Zhu Y, Liu F, Chen X, Wei L, Zhang N, Zhou Q, Zhong H, Yao C, Zhu X, Gong C, Zhu S, Zou C (2019) Traditional Chinese medicine Ze-Qi-Tang formula inhibit growth of non-small-cell lung cancer cells through the p53 pathway. J Ethnopharmacol 234:180–188
Yamaguchi H, Wang HG (2001) The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 20(53):7779–7786
Zhou F, Du J, Wang J (2017) Albendazole inhibits HIF-1α-dependent glycolysis and VEGF expression in non-small cell lung cancer cells. Mol Cell Biochem 428(1–2):171–178
Zhou H, Li XM, Meinkoth J, Pittman RN (2000) Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol 151(3):483–494
Acknowledgements
This work was financially supported by the Education Department of Anhui Natural Science Research Project China under Grant (KJ2018A0232, KJ2019A0326); the Foundation of Bengbu Medical College (Byycx1911).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 271 kb)
Rights and permissions
About this article
Cite this article
Ma, H., Bai, X., Sun, X. et al. Anti-cancer effects of methanol-ethyl acetate partitioned fraction from Magnolia grandiflora in human non-small cell lung cancer H1975 cells. J Bioenerg Biomembr 52, 175–183 (2020). https://doi.org/10.1007/s10863-020-09828-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-020-09828-6