Abstract
Mitochondrial reactive oxygen species (ROS) metabolism is unique in that mitochondria both generate and scavenge ROS. Recent estimates of ROS scavenging capacity of brain mitochondria are surprisingly high, ca. 9-12 nmol H2O2/min/mg, which is ~100 times higher than the rate of ROS generation. This raises a question whether brain mitochondria are a source or a sink of ROS. We studied the interaction between ROS generation and scavenging in mouse brain mitochondria by measuring the rate of removal of H2O2 added at a concentration of 0.4 μM, which is close to the reported physiological H2O2 concentrations in tissues, under conditions of low and high levels of mitochondrial H2O2 generation. With NAD-linked substrates, the rate of H2O2 generation by mitochondria was ~50–70 pmol/min/mg. The H2O2 scavenging dynamics was best approximated by the first order reaction equation. H2O2 scavenging was not affected by the uncoupling of mitochondria, phosphorylation of added ADP, or the genetic ablation of glutathione peroxidase 1, but decreased in the absence of respiratory substrates, in the presence of thioredoxin reductase inhibitor auranofin, or in partially disrupted mitochondria. With succinate, the rate of H2O2 generation was ~2,200–2,900 pmol/min/mg; the scavenging of added H2O2 was masked by a significant accumulation of generated H2O2 in the assay medium. The obtained data were fitted into a simple model that reasonably well described the interaction between H2O2 scavenging and production. It showed that mitochondria are neither a sink nor a source of H2O2, but can function as both at the same time, efficiently stabilizing exogenous H2O2 concentration at a level directly proportional to the ratio of the H2O2 generation rate to the rate constant of the first order scavenging reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The metabolism of reactive oxygen species (ROS) has been robustly documented to be abnormal in many neurodegenerative diseases, but the underlying mechanism is still unknown. Specifically, more data are needed to elucidate to which extents these abnormalities result from elevated ROS production or from a failure to scavenge ROS generated elsewhere. Whereas the sites of ROS generation in mitochondria are well known and the regulation of ROS production by the metabolic state of mitochondria is reasonably well understood (reviewed in (Andreyev et al. 2005; Starkov 2008), mitochondrial ROS scavenging remains, to a large extent, enigmatic. The enzyme composition of mitochondrial ROS scavenging system is known, its dependence on the NADPH and glutathione is well documented (Andreyev et al. 2005; Starkov 2008), but the capacity of this system and its interaction with ROS generation needs to be clarified. Two studies (Zoccarato et al. 2004; Drechsel and Patel 2010)provided new and important information about mitochondrial ROS scavenging system. The earlier study (Zoccarato et al. 2004) demonstrated that rat brain mitochondria can remove exogenously added H2O2 at high rates of ~ 0.3 – 6. 7 nmol/min/mg mitochondria protein, depending on the metabolic state of mitochondria and the nature of oxidative substrates. The highest rate of H2O2 removal (6.7 nmol/min/mg) was observed in mitochondria energized by NAD-linked substrates glutamate and malate. According to this study, mitochondrial glutathione reductase and glutathione peroxidase (GPx1) are the major players in removing exogenous H2O2 (Zoccarato et al. 2004). However, more recent study have challenged the importance of these two enzymes in H2O2 scavenging, placing the emphasis on mitochondrial ‘oxin enzymes (peroxiredoxins, thioredoxin, thioredoxin reductase) (Drechsel and Patel 2010). The authors have also reported very high rates of H2O2 scavenging by brain mitochondria, ca. 9–12 nmol H2O2/min/mg, which is ~100 times higher than the highest rate of ROS generation by brain mitochondria oxidizing NAD-linked (physiological) substrates (~0.06-0.1 nmol/min/mg, (Starkov 2008)). This creates a conundrum and raises a question whether brain mitochondria are, actually, a source, or a bona fide sink of ROS.
The rate of H2O2 removal in (Zoccarato et al. 2004) was measured by following the disappearance of a rather high amount of added H2O2 bolus (8 nmol in 1.6 ml, 5 μM H2O2 concentration). The rate of H2O2 removal in (Drechsel and Patel 2010) was measured by another method, but the pulse of H2O2 was also quite high (~3 μM, 3 nmol/ml). Although the highest reported level of H2O2 in brain stands as ~100 μM (Hyslop et al. 1995), conservative estimates of the steady-state levels of H2O2 in naive rat brain yielded much lower values, ~0.008 μM of H2O2 (Yusa et al. 1987), whereas typical steady-state H2O2 concentrations in other tissues and cell cultures are within the 0.01–0.1 μM range (Chance et al. 1979; Boveris and Cadenas 1997). In this study, we wanted to assess the properties of mitochondrial H2O2 scavenging system at H2O2 concentrations close to these low physiological ones. While most of the data obtained in our study are in a very good agreement with the results published by Patel’s group (Drechsel and Patel 2010), we think that the novel findings reported in this manuscript clarify the issue of “whether mitochondria are a source or a sink of ROS” question by demonstrating its irrelevance.
Materials and methods
The experiments were carried out in compliance with the National Institute of Health guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Cornell University. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Mouse brain mitochondria were isolated
by the Percoll gradient method as described (Sims 1990) with minor modifications. Animals were decapitated; the brains were excised and placed into ice-cold isolation buffer containing 225 mM mannitol, 75 mM sucrose, 20 mM HEPES-KOH (pH 7.4), 1 mM EGTA, and 0.5 g/l fatty acid-free bovine serum albumin (BSA). The cerebellum was removed, and the rest of the brain tissue was placed in a 15 ml Dounce homogenizer and homogenized manually with 20 strokes of tight-fitting pestle (“pestle A”). The brain homogenate was centrifuged at 1,250 × g for 5 min; the pellet was discarded and the supernatant was centrifuged at 14,000 × g for 10 min. The pellet was resuspended in 12 % Percoll (Sigma, St Louis, MO, USA) and layered on a preformed Percoll gradient (40 % and 23 %). Following centrifugation at 31,000 × g for 10 min, the mitochondrial fraction located at the interface of the 40 and 23 % Percoll layers was collected, diluted with the isolation buffer, and centrifuged at 14,000 × g for 10 min. The supernatant was discarded, and the loose pellet was resuspended in the isolation buffer and centrifuged at 12,000 × g for 10 min. The resulting pellet was resuspended in 100 μl of the isolation medium and stored on ice during the experiment.
The rate of H2O2 emission from mitochondria
was estimated by a fluorescence assay with Hitachi 7,000 (“Hitachi High-Tech”, Japan) spectrofluorimeter (exCitation, 555 nm; emission, 581 nm) as described earlier (Starkov and Fiskum 2003). Briefly, mitochondria (0.01–0.04 mg/ml) were placed in a magnetically stirred cuvette with 1 ml of respiratory assay buffer (125 mM KCl, 4 mM K2HPO4, 20 mM HEPES-KOH (pH 7.2), 0.2 mg/ml of BSA, 1 mM EGTA) containing respiratory substrates (either 5 mM glutamate + 2 mM malate, or 5 mM succinate), 10 μM Amplex® Ultrared (“Life Technologies”, Grand Island, NY, USA), 4 U/ml of horse radish peroxidase (HRP), and 20 U/ml of Cu, Zn superoxide dismutase. The calibration curve was obtained by adding 100pmol aliquots of freshly made H2O2 to the cuvette containing the respiratory assay buffer, Amplex® Ultrared, and HRP.
To measure H2O2 scavenging or accumulation
mitochondria were added at a 0.01 – 0.04 mg/ml concentration to 7 ml of the incubation buffer in a thermostated (t = 37 °C) stirred glass chamber protected from light. The incubation buffer contained 125 mM KCl, 4 mM K2HPO4, 20 mM HEPES-KOH (pH 7.2), 0.2 mg/ml of BSA, 1 mM EGTA, with or without respiratory substrates and other additions, as indicated in the legends to Figs. 1 and 2. For the H2O2 scavenging assay, mitochondria were incubated for 300 s; than a single bolus of 400 pmol/ml H2O2 was added. After incubating mitochondria for a 50–200 s with H2O2, 0.9 ml aliquot of the mitochondria suspension was withdrawn and transferred into the spectrofluorimeter cuvette containing the stirring bar and 100 μl of the same incubation buffer supplemented with Amplex® Ultrared, HRP, and Cu, Zn superoxide dismutase, and fluorescence was recorded immediately. The procedure was repeated until 5–6 different time data points were obtained. A typical recording illustrating this procedure is presented in Fig. 1a. The procedure to measure H2O2 accumulation in the mitochondria suspension was similar except no H2O2 was added (Fig. 2a).
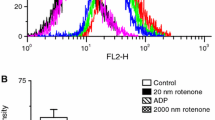
H2O2 scavenging by mouse brain mitochondria oxidizing glutamate and malate. A, a typical experiment to estimate ROS scavenging capacity (see “Methods”). Black circles indicate the data points (the H2O2 amount that remained in the mitochondria suspension at the indicated time) used to construct a graph presented on panel B. Mitochondria were added at -300 sec (“Methods”) to the H2O2 addition time point. B, H2O2 scavenging dynamics under various conditions. The point at “0 sec” was obtained by adding H2O2 to the assay medium in the absence of mitochondria. The dashed line indicates the time point used to estimate the remaining H2O2 in panels Cand D. C, the effect of various metabolic conditions on the H2O2 scavenging. The levels of H2O2 remaining in the suspension after 400 s incubation are shown. D, the effect of mitochondria structural integrity and thioredoxin reductase inhibition on the ROS scavenging capacity of mitochondria. Additions: rotenone was added at 1 μM, ADP at 1 mM, FCCP at 120 nM. Abbreviations: G:M, 5 mM glutamate and 2 mM malate were added as the respiratory substrates; “State 3”, the incubation medium was supplemented with 1 mM ADP; “no GpX1”, mitochondria were isolated from brains of mice with genetically ablated glutathione peroxidase 1; “frozen-thawed”, mitochondria were frozen and thawed to disrupt their structural integrity and incubated in the absence of added oxidative substrates. The number of experiments was n = 8 (“G:M” and “G:M + rotenone”) and n = 4 for all other conditions except “G:M, no GPx1” (n = 2)
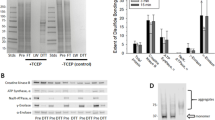
H2O2 accumulation and scavenging by mouse brain mitochondira oxidizing succinate. A, typical experiment to estimate ROS accumulation in the medium containing mitochondira oxidizing succinate (see “Methods”). Black circles indicate the data points (the H2O2 amount that was accumulated in the mitochondria suspension at the indicated time) used to construct the graphs presented on panels B and C. B, the H2O2 accumulation dynamics at different concentrations of mitochondria protein. Dashed lines indicate the dynamics of H2O2 accumulation calculated by the Eq. 3 (see the text). C, the data from B after normalizing the amount of H2O2 by the protein content in the assay. D, typical H2O2 scavenging dynamics with succinate-oxidizing mitochondria. White triangles and squares indicate the amount of H2O2 remaining in the assay medium at the indicated time points. The points at “0 sec” were obtained by adding H2O2 to the assay medium in the absence of mitochondria
Immunoblotting for catalase in isolated brain mitochondria
To determine the amount of catalase present in mitochondria, we loaded 2.5, 6.25, 12.5, 18.75, 25 ng of calalase (“Sigma”, USA, cat. # C40) and 60–200 microgram of isolated mouse brain mitochondria in 4–20 % gel. Protein were resolved by SDS-PAGE, transferred onto PVDF membrane and stained with monoclonal anti-catalase antibody (C0979, Sigma). Membranes were then incubated with HRP-conjugated secondary antibody (Jackson ImmunoResearch) and visualized using SuperSignal West Pico Chemiluminescent Substrate (Thermo). The amount of protein was quantified with ImageJ software (NIH, USA); catalase samples were used to build the calibration curve. The amount of catalase in brain mitochondria was determined using this calibration curve.
Statistical analysis (t-test with non-parametric homoscedastic distribution settings) was performed with the help of Excel software Statistical Analysis package (Microsof Office 2010, Microsoft, USA).
Results
H2O2 scavenging at low rates of H2O2 generation by mitochondria
To avoid the interference from mitochondria-generated H2O2, we used experimental conditions that provided very low generation of H2O2. Mitochondria fueled by glutamate and malate in the absence of other additions were generating H2O2 at a rate of 75 ± 10 pmol/min/mg that was steady over 1,200 s of the recording time. In the presence of rotenone, mitochondria generated H2O2 at a rate of 190 ± 20 pmol/min/mg. Both observed rates were within the earlier reported range for brain mitochondria oxidizing NAD-linked substrates (Andreyev et al. 2005). Therefore, even in a complete absence of any scavenging, under our conditions (mitochondria concentration, 0.01 mg protein/ml), mitochondria could not have generated more than 15 or 38 pmol (in the absence or presence of rotenone, respectively) of H2O2 within 20 min of the assay duration. These amounts are considerably lower (~4 and 10 %) than the amount of H2O2 (400 pmol/ml) added to the mitochondria to study H2O2 scavenging dynamics. Figure 1A illustrates the procedure of measuring the scavenging of H2O2. The influence of the mitochondrial functional state on the H2O2 scavenging was assessed by imposing several well-defined experimental conditions: the “resting state (State 4)” (glutamate + malate, no other additions), “permanent phosphorylation state (State 3)” (1 mM ADP was added), “complete uncoupling” (120 nM FCCP was added), “de-energized” (no glutamate and malate was added), and “no structural integrity” (mitochondria were frozen and thawed two times, washed by diluting to 0.5 mg/ml in the incubation buffer without substrates, centrifuged at 10,000 g × 10 min and re-suspended to 5 mg/ml). The typical results of H2O2 scavenging experiment are shown in Fig. 1B. The dynamics of H2O2 scavenging was non-linear; the best fit (R2 > 0.98, using Microsoft Office Excel in-built “trend line” function) to these data points was obtained with an exponential function of the type (Eq. 1):
where [H2O2](t) is the concentration of H2O2 remaining in the solution at the time “t”, [H2O2](t=0) is the amount of H2O2 added to the mitochondria, “t” is time, “e” is the base of the natural logarithm, and “k” is a rate constant of the H2O2 scavenging reaction. Thus, the scavenging reaction at the used H2O2 concentration is a first order reaction and therefore its rate should not be expressed in “nmol/min/mg”, because the rate of scavenging depends on the concentration of the substrate (H2O2). There was no consistent fit to the data with exponential function with frozen-thawed mitochondria. The rate constant of the scavenging reaction was estimated with glutamate + malate in the absence of other additions, it was 0.002 ± 0.0004 (n = 8). For the data presented in Fig. 1C, the rate constants were within the 1.06 × 10−3 – 2.7 × 10−3 range (except with frozen-thawed mitochondria). Since visual comparison of the rate constants is not immediately illustrative of the changes in the scavenging capacity, we have selected a time point at which approximately half of the added H2O2 was decomposed (dashed line in Fig. 1B), which allowed us to illustrate the effects of various mitochondria metabolic conditions on the H2O2 scavenging capacity (Fig. 1C and D). We found that de-energizing mitochondria by uncoupling or putting them in a permanent active phosphorylation state (State 3, “glutamate + malate + ADP”, Fig. 1C) did not affect their H2O2 scavenging capacity. The absence of mitochondrial glutathione peroxidase 1 (GPx1) also did not affect the H2O2 scavenging capacity (the GPx1 knockout mice were generously provided by Prof. Ye-Shi Ho, Department of Biochemistry, Wayne State University, Detroit, Michigan). The scavenging capacity of mitochondria was impaired by inhibiting Complex I of the respiratory chain (which also increased the generation of H2O2, see above), depriving mitochondria of the respiratory substrates, or disrupting the structural integrity of mitochondria (“frozen-thawed”, Fig. 1C). Inhibiting thioredoxin reductase with auranofin also diminished the H2O2 scavenging capacity to some extent, more so in the frozen-thawed mitochondria (Fig. 1D), although the effect was not as pronounced as reported earlier (Drechsel and Patel 2010). The absence of pronounced effects of substrates and mitochondria energization on the scavenging of H2O2 may be explained by the presence of some energy-independent H2O2 scavenging enzyme, such as catalase. The latter had been shown in heart and liver mitochondria, but there are no reports on the presence of catalase in brain mitochondria. We therefore have checked for catalase in isolated Percoll-purified brain mitochondria by immunostaining (see “Methods”). We have found that mouse brain mitochondria contain 72.2 ± 0.8 ng catalase per mg of mitochondrial protein. This would amount to ~1.2 μM catalase concentration in the matrix (assuming that the matrix volume is about 1 μl per mg of mitochondria protein, the molecular mass of catalase’s catalytically active monomer is about 60 kD, and that it is located in the matrix and not a contamination from peroxisomes co-isolated with mitochondria). Considering such low abundance and the fact that catalase inhibitor 3-amino-1,2,4-triazole had no effect on the scavenging capacity of brain mitochondria (Drechsel and Patel 2010) and results of this study, data not presented), we have decided not to pursue this any further.
H2O2 scavenging at high rates of H2O2 generation by mitochondria
The experiments were performed with mitochondria oxidizing succinate in State 4. Under these conditions, H2O2 is generated in the reaction of reverse electron transfer from succinate dehydrogenase to Complex I and to the matrix-located H2O2 generating enzymes (e.g., alpha-ketoglutarate and pyruvate dehydrogenase complexes) (Andreyev et al. 2005). In our experiments, mitochondria fueled by succinate in the absence of other additions were generating H2O2 at a rate of 2,682 ± 252 pmol/min/mg (at 0.01 mg/ml mitochondria concentration; see the “Discussion” section). In the absence of scavenging, this should have resulted in the accumulation of ~270 pmol of H2O2 in the assay medium within 10 min. Indeed, we detected about that amount of H2O2 after 10 min of incubation (Fig. 2B); but at 16 min, the accumulation was much less than would be expected. Moreover, with higher amount of mitochondrial protein (0.02 and 0.04 mg/ml), the accumulation rates were considerably lower that the expected values (Fig. 2B). Thus, the amount of accumulated H2O2 at the mitochondria concentration of 0.04 mg/ml should have exceeded 900 pmol, but reached only 420 pmol in our experiment (Fig. 2B). This indicates a substantial rate of H2O2 scavenging by succinate-oxidizing mitochondria, which is even more evident when the amount of accumulated H2O2 is normalized by the added mitochondria protein (Fig. 2C; note that the highest H2O2 accumulation per mg protein was observed with 0.01 mg of mitochondria). It interesting that succinate-oxidizing mitochondria were apparently not efficient in scavenging of externally added H2O2 (Fig. 2D); the scavenging was practically absent with 400 pmol/ml H2O2 pulse and marginal with 750 pmol/ml H2O2 pulse (Fig. 2D).
Discussion
In general, the properties of brain mitochondria H2O2 scavenging system observed in our experiments with NAD-linked substrates are in a very good agreement with those published by (Drechsel and Patel 2010). That is, the scavenging efficacy depends on the substrates, can be diminished by thioredoxin reductase inhibitors, and apparently does not require mitochondrial glutathione peroxidase (Fig. 1C). The effects observed were much smaller than those reported in (Drechsel and Patel 2010), most likely because under our conditions, the scavenging system was much less dependent on the energy production in mitochondria due to the relatively large pools of required co-factors and scavenging enzymes as compared to the size of the H2O2 bolus. As was mentioned above, mitochondria contain ~2–5 nmol/mg NAD(P)H, about the same amount of glutathione, which is in more than 90 % in the reduced state, and abundant peroxiredoxins. The concentration of peroxiredoxin 3 in the mitochondrial matrix is estimated ca. 60 μM, that of peroxiredoxin 5 is ca. 20 μM, whereas the concentration of glutathione peroxidase 1 is only ~ 2 μM (Cox et al. 2010). The new finding in this work is that the scavenging capacity is apparently independent on the workload of mitochondria, such as oxidative phosphorylation of ADP, and that it is not affected by uncoupling of mitochondria (Fig. 1C), at least at low concentrations of H2O2. Another novel finding is that H2O2 scavenging reaction can be characterized by the first order equation, which makes possible to generate a simple model linking mitochondrial H2O2 production to scavenging, and thereby allows estimation of H2O2 steady-state levels in mitochondria-containing environment. Mitochondria both produce and scavenge H2O2; the overall reaction can be described in general terms as (Eq. 2).
where “V” is the rate of H2O2 production (expressed in pmols H2O2/s) and “k” is the scavenging reaction rate constant. Assuming that the rate of H2O2 production by mitochondria is constant (unless their metabolic conditions change) and that, as we found, the scavenging reaction is of the first order type ([H2O2](t) = [H2O2](t=0) × e-k t), this equation can be solved as (Eq. 3).
where [H2O2](t) is H2O2 concentration (in pmol/ml) in the medium at the time t (in seconds), V is the rate of H2O2 production, and k is the scavenging reaction rate constant. At t > ∞ (at the “steady state”), the equation is transformed to \( {\left[{\mathrm{H}}_2{\mathrm{O}}_2\right]}_{(t)}=\frac{V}{k} \) This allows us to find the scavenging rate constant from the data presented in Fig. 2B. Assuming that H2O2 accumulation has reached the steady state at 800 sec (mitochondrial concentration, 0.04 mg/ml; H2O2 amount at 800 s was 455 pmol; Fig. 2B), and using the rates of H2O2 production measured in the representative experiments shown in Fig. 2B (2,916 pmol/min/mg with 0.01 mg/ml, 2,706 with 0.02 mg/ml, and 2,262 with 0.04 mg/ml mitochondria), the scavenging rate constants for 0.01, 0.02, and 0.04 mg/ml mitochondria concentrations were 1.07 × 10−3, 1.98 × 10−3, and 3.32 × 10−3, correspondingly. Using these numbers and the equation 3, we calculated the dynamics of H2O2 accumulation (dashed lines in Fig. 2B). In our opinion, the correlation between the calculated and measured values is sufficiently good to prove that our model is correct in general. It explains why succinate-oxidizing mitochondria were apparently less efficient in scavenging of the added H2O2 bolus (Fig. 2D) than mitochondria oxidizing NAD-linked substrates (Fig. 1): the H2O2 accumulation resulting from mitochondrial H2O2 generation was much higher with succinate than with glutamate + malate. To this end, it should be noted that upon succinate oxidation, the scavenging system is most likely receiving an adequate supply of reduced pyridine nucleotides for normal functioning, because higher levels of NAD(P)H were found in mitochondria energized with succinate compared with complex I substrates (c.f. from Adam-Vizi and Chinopoulos (2006)).
Overall, the data demonstrate that mitochondria stabilize H2O2 steady-state concentration in the surrounding medium (e.g., in cell cytosol) at the level determined by the V/k ratio. Mitochondria are a source of H2O2 when the H2O2 concentration is below that steady state level, or they are bona fide sink of H2O2 when H2O2 concentration exceeds that level, efficiently dampening the pulses of exogenously produced H2O2. In turn, the V/k ratio would be determined by the abundance of mitochondrial antioxidant enzymes (the “k” value) and metabolic conditions, such as the nature of substrates and the workload of mitochondria, as these conditions affect the rate of mitochondrial H2O2 generation (the “V”). Thus, mitochondria are capable of adjusting the steady-state level of H2O2 according to current cellular conditions, such as mitochondria abundance, changes in the intracellular metabolic demands, chemical nature and availability of oxidative substrates, and external ROS “pressure”. Thereby, mitochondria serve as a “ROS-stabilizing device” in the cellular ROS signaling network, as we have proposed earlier (Starkov 2008).
References
Adam-Vizi V, Chinopoulos C (2006) Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci 27(12):639–645. doi:10.1016/j.tips.2006.10.005
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70(2):200–214
Boveris A, Cadenas E (1997) Cellular sources and steady-state levels of reactive oxygen species. In: Clerch LB, Massaro DJ (eds) Oxygen, gene expression and cellular function. Marcel Dekker, New York, pp 1–25
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59(3):527–605
Cox AG, Winterbourn CC, Hampton MB (2010) Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem J 425(2):313–325. doi:10.1042/BJ20091541
Drechsel DA, Patel M (2010) Respiration-dependent H2O2 removal in brain mitochondria via the thioredoxin/peroxiredoxin system. J Biol Chem 285(36):27850–27858. doi:10.1074/jbc.M110.101196
Hyslop PA, Zhang Z, Pearson DV, Phebus LA (1995) Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res 671(2):181–186
Sims NR (1990) Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem 55(2):698–707
Starkov AA (2008) The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 1147:37–52. doi:10.1196/annals.1427.015
Starkov AA, Fiskum G (2003) Regulation of brain mitochondrial H2O2 production by membrane potential and NAD (P) H redox state. J Neurochem 86(5):1101–1107
Yusa T, Beckman JS, Crapo JD, Freeman BA (1987) Hyperoxia increases H2O2 production by brain in vivo. J Appl Physiol 63(1):353–358
Zoccarato F, Cavallini L, Alexandre A (2004) Respiration-dependent removal of exogenous H2O2 in brain mitochondria: inhibition by Ca2+. J Biol Chem 279(6):4166–4174
Acknowledgment
This work was supported by the National Institutes of Health/National Institute on Aging grant PO AG 14930 to A.A.S., and in part by the grants from Ministry of Education and Science of Russian Federation (State assessment number 149.2014/K) and the Russian Scientific Fund (number 14-14-00181) to V. N.P. and Russian Fund for Basic Research grant 14-04-316118 to M.S.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starkov, A.A., Andreyev, A.Y., Zhang, S.F. et al. Scavenging of H2O2 by mouse brain mitochondria. J Bioenerg Biomembr 46, 471–477 (2014). https://doi.org/10.1007/s10863-014-9581-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-014-9581-9