Abstract
Infections of vascular prostheses are still a major risk in surgery. The current work presents an in vitro evaluation of novel slow release antibiotic coatings based on new gentamicin fatty acid salts for polytetrafluoroethylene grafts. These grafts were coated with gentamicin sodium dodecyl sulfate, gentamicin laurate and gentamicin palmitate. Drug release kinetics, anti-infective characteristics, biocompatibility and haemocompatibility of developed coatings were compared to commercially available gelatin sealed PTFE grafts (SEALPTFE™) and knitted silver coated Dacron® grafts (InterGard®). Each gentamicin fatty acid coating showed a continuous drug release in the first eight hours followed by a low continuous release. Grafts coated with gentamicin fatty acids reduced bacterial growth even beyond pathologically relevant high concentrations. Cytotoxicity levels depending on drug formulation bringing up gentamicin palmitate as the most promising biocompatible coating. Thrombelastography studies, ELISA assays and an amidolytic substrate assay confirmed haemocompatibility of developed gentamicin fatty acid coatings comparable to commercially available grafts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biomaterials are designed in an ideal case to replace the human anatomy. Bacterial infections related to biomaterials are an undeniable complication of modern surgery. Still implant-associated infections pose a special and complex challenge and lead to surgical interventions. In the case of vascular surgery such a complication means a direct life-threatening risk [1].
Vascular surgery offers a variety of vascular grafts to replace obstructed, dilated or traumatised vascular segments. The saphenous vein is still considered to be the best autogenous graft material for arterial bypasses below the inguinal ligament and represents the “gold standard” [2]. The implantation of alloplastic vascular prostheses made of Dacron® (polyethylene terephthalate) or ePTFE (expanded polytetrafluorethylene) is indicated, when a venous graft is not available.
Periprosthetic infections, however, pose a direct threat to the extremity and the life of the patient. According to Vollmar et al. [3], the risk for deep wound infection averages at 2.6 %, while other authors report rates up to 11 % of which 67 % were involving the graft. One quarter of all patients who were affected by infections experienced the loss of an extremity, and 11 % died from infection [4]. Graft infections have to be treated surgically by removing the infected graft and a radical debridement is necessary to reduce bacterial contamination and provide the recovery of the blood supply [1].
Bacteria which are responsible for infections and most frequently observed were gram-positive staphylococci, Staphylococcus aureus and S. epidermidis, as well as gram-negative germs like Escherichia coli or pseudomonades [4–6].
In order to prevent infections, surgeons have established different strategies to reduce bacterial contamination. Besides operating techniques, especially the perioperative systemic administration of antibiotics and the local application of antibiotics have shown a remarkable improvement in the reduction of infections [7, 8]. For this purpose it is essential that the used local acting antibiotics are able to prevent bacterial colonisation and formation of biofilms at the surface of the prosthesis by generating a temporary high local drug level. Moreover, a delayed drug release from the surface of the prosthesis to the adjacent tissue is desired.
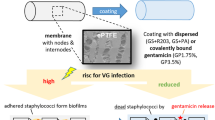
In this study we focused on the development of novel antibiotic coatings for vascular prostheses. As described above a modern vascular graft and its coating must meet the demand of limited host response, high patency and a reliable antibiotic protection. The performed in vitro evaluation characterised the antibiotic release rates, haemocompatibility, cytotoxicity and antimicrobial effectiveness of three new gentamicin coatings. These coatings show a delayed release of the incorporated antibiotics reliably avoiding a colonisation by human pathogenic bacteria such as S. epidermidis. This technology might be of high clinical interest for the prevention or at least reduction of the number of infected patients after receiving an implant.
2 Materials and methods
2.1 Medical implants
Expanded PTFE grafts Alpha Graft® PTFE (Alpha Research Deutschland GmbH, Berlin, Germany), gelatin sealed ePTFE grafts SEALPTFE™ (Vascutek Deutschland GmbH, Hamburg, Germany) and knitted silver coated PET grafts InterGard® (InterVascular, La Ciotat, France) were used in the present study—all of 6 mm inner diameter.
2.2 The anti-infective coating
Equipments consisting of newly developed gentamicin salts were prepared for the anti-infective coating of Alpha Graft® PTFE grafts. Gentamicin sodium dodecyl sulfate (Genta-SDS), gentamicin laurate (Genta-L) and gentamicin palmitate (Genta-P) were provided by Heraeus Medical GmbH, Wehrheim, Germany. Gentamicin fatty acid salts were prepared according to Vogt et al. [9]. The mass percentage of gentamicin base in the respective salts was specified at 25 % for Genta-SDS, at 31 % for Genta-L and at 26 % for Genta-P. A mass of 250 mg of each gentamicin salt were solved in 15 ml of a chloroform/methanol mixture (Sigma-Aldrich AG, Deisenhofen, Germany). Ratios for chloroform/methanol mixture diversify because of different dilution characteristics of 11:1 in case of Genta-SDS and Genta-L and of 5:1 in case of Genta-P.
With regard to the anti-infective equipment of SEALPTFE™ grafts, a rifampicin solution (Eremfat® i.v. 600 mg, Fatol GmbH, Schiffweiler, Germany) was prepared in accordance to the instructions for use provided by Vascutek GmbH.
2.3 Coating process for non-commercial available coatings
Alpha Graft® PTFE grafts were coated with the coating solutions mentioned above by a dip-coating procedure ensuring a uniformly distributed anti-infective coating. The dip-coating procedure was carried out in sterile sealable glass vials for 30 min followed by a loft drying for 10 min. Coating and drying procedures were carried out under aseptic conditions in a laminar air-flow hood. The concentration of each coating was assessed by determining the weight difference of grafts before and after the coating procedure. This method enables to equip ePTFE grafts in the middle with up to 1–2 mg gentamicin salts per cm length.
2.4 Morphological analysis (SEM)
Coated Alpha Graft® PTFE grafts were prepared for SEM by sputtering with Gold (BAL-TEC MED 020 coating system, Boeckeler Instruments, Arizona, USA) and examined with a high vacuum SEM (JSM 6060LV Scanning Electron Microscope, JEOL Ltd., Tokyo, Japan).
2.5 Antibiotic release
Drug release from coated Alpha Graft® PTFE grafts with 1 cm length (n = 3) was studied in 2 ml phosphate-buffered saline (PBS, pH 7.4) at 37 °C and 300 rpm in a thermomixer (Eppendorf, Hamburg, Germany). Uncoated Alpha Graft® PTFE grafts served as control. At time intervals 15 min, 30 min, 1 h, 4 h, 8 h and 24 h, the elution medium was completely changed. Samples were analysed for gentamicin via TDx Assay (Abott Laboratories, Il, USA).
2.6 Provoked Infections with S. epidermidis
For in vitro studies a clinical isolate of biofilm developing S. epidermidis (ATCC® 35984) was used [10]. The test strain was susceptible to gentamicin (MIC 0.5 mg/l) and cultured on Mueller–Hinton II agar plates (Becton–Dickinson GmbH, Heidelberg, Germany) at 37 °C for 24 h before testing.
2.7 Antibacterial characteristics
2.7.1 Anti-infective efficiency of grafts via drug release
In order to investigate the antimicrobial potential of coated Alpha Graft® PTFE grafts, coated SEALPTFE™ grafts and InterGard® grafts (n = 3), grafts were eluted for 2 h in 10 ml PBS at 37 °C at 300 rpm in a thermomixer. The eluates gained were inoculated with 10,000 colony forming units (cfu) of S. epidermidis per ml and incubated for 2 h at 37 °C. Subsequently, 100 μl of the resulting eluates were plated on Mueller–Hinton II agar plates, incubated for another 24 h followed by a visual determination of cfu.
S. epidermidis suspensions of 1,000, 5,000 and 10,000 cfu/ml were prepared in Tryptone Soya Broth (BD, Heidelberg, Germany) via densitometry of bacterial suspensions to a McFarland value of 0.5 (5 × 107 cfu/ml). Uncoated and coated Alpha Graft® PTFE grafts, coated SEALPTFE™ grafts, InterGard® grafts and additionally 2 h preeluted coated Alpha Graft® PTFE grafts—each of 1 cm length (n = 4, for each bacterial concentration and each coating type) were incubated in 2 ml of these bacterial suspensions for 18 h at 37 °C. Subsequently, grafts were removed from bacterial suspensions. A volume of 100 μl of the resulting eluates were plated out on Mueller–Hinton II agar plates, incubated for another 24 h followed by a visual determination of cfu.
2.8 Adhesion of viable bacteria
In order to determine the number of pathogens attached, removed grafts mentioned above were subsequently washed twice with isotonic saline and placed into an ultrasonic bath for 12 min. A volume of 100 μl of bacterial suspensions obtained were plated on Mueller–Hinton II agar plates. Agar plates were incubated for 18 h at 37 °C and cfu were counted to get number of adsorbed pathogens.
2.9 Cell culture
Mouse connective tissue fibroblasts L929 were cultured in RPMI 1640 (Sigma-Aldrich Chemie GmbH, Munich, Germany) medium supplemented with 10 % fetal bovine serum, penicillin and streptomycin (100 U/ml and 0.1 mg/ml, respectively), partricin (50 μg/ml) and stable glutamine at 37 °C in a humidified atmosphere containing 5 % CO2.
2.10 In vitro cytotoxicity studies
Cytotoxicity studies were performed with gentamicin salt coated Alpha Graft® PTFE grafts, coated SEALPTFE™ grafts and InterGard® grafts (n = 8, for each coating type) regarding to the ISO standard 10993. In order to achieve the optimal ratio between the surface area of the grafts and the area of cell culture, grafts were cut into 1.0 mm2 pieces. Subsequently, cells were cultured in 96-well microplates in presence of coated grafts and immersed in 200 μl medium. Cells were cultured until subconfluence, grafts were removed from the microplates and metabolic activity of viable cells was measured with cell proliferation reagent WST-1 (Roche Diagnostics GmbH, Mannheim, Germany).
2.11 Haemocompatibility studies
2.11.1 Thrombelastography studies
In order to investigate the influence of gentamicin salt coatings on haemostasis, blood coagulation was investigated globally by thrombelastography, measuring the clot formation time (CT) and the maximal clot firmness (MCF) via a ROTEM® analyser (Pentapharm GmbH, Munich, Germany).
Therefore, aluminium cups and pins were fabricated suitable for use in a ROTEM® system and coated with gentamicin solutions as described before. The amount of coating was determined by weight difference of cups and pins before and after the coating procedure. Thrombelastography studies for whole blood were carried out using the in-tem® (Pentapharm GmbH, Munich, Germany) for analysing the intrinsic pathway of blood coagulation. Therefore, 300 μl of freshly drawn citrate anticoagulated human whole blood was given in each cup and 10 μl in-tem® solution (Pentapharm GmbH, Munich, Germany) was added. Followed by adding 10 μl star-tem® (Pentapharm GmbH, Munich, Germany) recording was started.
2.12 Blood coagulation markers
In order to study thrombogenic characteristics of biomaterials, uncoated and coated vascular prostheses were incubated with freshly drawn non anticoagulated human blood. For that purpose, Alpha Graft® PTFE grafts, coated SEALPTFE™ grafts and InterGard® grafts (n = 2, length 8 cm for each coating type) were fixed in 50 ml-tubes (Carl Roth, Karlsruhe, Germany) and locked with an adjusted Combifix adapter (neoLab, Heidelberg, Germany) (Fig. 1). This allowed a filling of grafts with 2 ml of freshly drawn human whole blood. Incubation was stopped after 7 min by anticoagulation with trisodium citrate or EDTA. In order to get information about clotting activation, blood markers of coagulation were determined after generating plasma. Citrated blood plasma was examined with an amidolytic substrate assay for factor XIIa-like activity (Unitest™ FXIIA, Unicorn Diagnostics Ltd, Kent, UK) as well as by monoclonal enzyme immunoassays for plasma F1+2 values (Enzygnost F1+2 micro, Behring, Marburg, Germany). EDTA blood plasma was investigated for C3a-desArg (Complement C3a-desArg ELISA, Progen Biotechnik GmbH, Heidelberg, Germany).
3 Results
3.1 Coated grafts are not altered concerning porosity
Gentamicin fatty acid salt coatings of Alpha Graft® PTFE grafts were investigated through SEM after dip-coating. All coated grafts kept their flexibility and open pores were detected for both side penetrations (Fig. 2).
3.2 Gentamicin is released over a time period of 24 h
The content of various gentamicin salts determined at 1–2 mg/cm Alpha Graft® PTFE graft (Genta-SDS = 1.08 mg/cm ± 0.16, Genta-L = 1.07 mg/cm ± 0.12, Genta-P = 1.12 mg/cm ± 0.11) representing a coating of approximately 0.3 mg gentamicin each 1 cm of Alpha Graft® PTFE graft averaged at 0.27 mg for Genta-SDS, at 0.33 mg for Genta-L and at 0.29 mg for Genta-P (Table 1).
To obtain information about time dependant drug kinetics, release rates were defined as the percentage of released gentamicin with respect to the total amount of gentamicin in the coatings.
Each gentamicin fatty acid combination showed a continuous drug release during first 8 h followed by a low release of gentamicin for the following 16 h. Genta-L and Genta-P showed a comparable drug release after the first hour of about 30 % while Genta-SDS showed an average release of 17 %. In the second hour of release, Genta-L showed the highest released gentamicin amount of about 50 % followed by Genta-P of about 40 % and Genta-SDS of about 35 %. After two more hours of release Genta-L and Genta-SDS averaged at 70 % whereas the amount of released gentamicin from Genta-P averaged at 58 %. After a total release time of 8 h Genta-SDS showed the highest released gentamicin amount of 95 % followed by Genta-L of 82 % and Genta-P of 70 %. In the following 16 h only small amounts of gentamicin were released for each gentamicin salt coating. Genta-SDS reached a release rate of 107 %, Genta-L of 87 % and Genta-P 75 % after 24 h release interval. These data demonstrate that gentamicin formulation of fatty acid salts shows a retarded release characteristic (Fig. 3).
3.3 Gentamicin coated grafts inhibit bacterial growth
3.3.1 Anti-infective efficiency of grafts is achieved via drug release
SEALPTFE™ graft eluates showed no colonies of S. epidermidis on incubated Mueller–Hinton II agar plates obtained from rifampicin soaked gelatin coated grafts eluted for 2 h concluding total germ eradication.
Genta-SDS, Genta-P and silver knitted grafts (InterGard®) showed comparable results with regard to the antibacterial efficiency of 2 h eluates inoculated with 104 cfu/ml of S. epidermidis over 2 h. Counts of cfu on Mueller–Hinton II agar plates for eluates obtained from these grafts revealed a remaining S. epidermidis concentration of 2.4 × 102 cfu/ml (Genta-SDS), 2.0 × 102 cfu/ml (Genta-P) and 2.6 × 102 cfu/ml for InterGard® grafts. The lowest antibacterial efficiency was shown by eluates obtained from Genta-L grafts with an average concentration of 4.4 × 102 cfu/ml. Eluates obtained from uncoated reference grafts developed an average concentration of 105 cfu/ml.
With regard to antibacterial efficiency of grafts being in direct contact with S. epidermidis suspensions of different concentrations (1,000, 5,000 and 10,000 cfu/ml) for 18 h, the visual determination of S. epidermidis colonies on Mueller–Hinton II agar plates incubated with the resulting suspension from SEALPTFE™ grafts and InterGard® grafts revealed a complete eradication of S. epidermidis irrespective of the bacterial concentration.
Genta-SDS showed a complete eradication of pathogens after 18 h of incubation irrespective of the tested bacterial concentrations. Genta-P showed a complete eradication of pathogens after 18 h of incubation only for the initial S. epidermidis concentration of 1,000 cfu/ml. At 5,000 and 10,000 cfu/ml, only two of four suspensions were evaluated and were free of pathogens whereas at an initial bacterial concentration of 5,000 cfu/ml one suspension showed a bacterial concentration of about 500 cfu/ml, the other one of about 3,000 cfu/ml and at an initial bacterial concentration of 10,000 cfu/ml two suspensions showed a bacterial concentration of about 500 cfu/ml each.
Minor antibacterial efficiency was determined for Genta-L coated grafts. Starting at the lowest initial bacterial concentration of 1,000 cfu/ml, in two of four suspensions evaluated, S. epidermidis colonies were determined at bacterial concentrations of about 500 cfu/ml and about 3,000 cfu/ml, respectively. Similar results were observed for initial bacterial concentrations of 5,000 and 10,000 cfu/ml with the difference that for the initial bacterial concentration of 10,000 cfu/ml a higher pathogen contamination of resulting suspensions was determined. All suspensions obtained from uncoated reference grafts showed a bacterial contamination.
3.4 Adhesion of viable bacteria onto the vascular graft
The determination of pathogens attached to graft surfaces after incubation in bacterial suspensions revealed that original and preeluted Genta-SDS, Genta-P, Genta-L and coated SEALPTFE™ grafts showed no adhesion of pathogens on their surface irrespective of the tested initial bacterial concentration. In these experiments InterGard® grafts could not provide a protection from pathogen adherence when incubated with higher initial S. epidermidis concentrations (i.e. 10,000 cfu/ml).
3.5 Viability of L929 mouse fibroblasts is not markedly influenced by Genta-P and Genta-L
Related to reference cell’s metabolic activity after 1.5 h of incubation the WST-1 assay showed the highest activity of viable L929 fibroblasts in presence of Alpha Graft® PTFE grafts coated with Genta-L (100 %) followed by SEALPTFE™ and Genta-P grafts (90.8 %) and InterGard® grafts (77.2 %). Cells in presence of Alpha Graft® PTFE grafts coated with Genta-SDS showed a very low metabolic activity demonstrating a high cytotoxic potential (10.5 %).
3.6 Coated grafts show haemocompatible properties comparable to uncoated grafts
3.6.1 Thrombelastography studies
All cuvettes coated with the various gentamicin salts showed increased CT values compared to uncoated cuvettes. CT values for Genta-P averaged at 166 ± 48 s followed by those for Genta-SDS at 254 ± 148 s. Genta-SDS coated cuvettes caused results in a range of 230–1,700 s. CT values for uncoated reference cuvettes averaged at 152 ± 52 s (Table 2).
With regard to MCF values, the lowest MCF value within the group of gentamicin salt coated cuvettes was shown by Genta-SDS at 12 mm, followed by Genta-L at 33 ± 10 mm and Genta-P at 56 ± 12 mm. MCF values for uncoated reference cuvettes averaged at 50 ± 11 mm. Both parameters demonstrated an anticoagulation effect of investigated coatings.
3.7 Blood coagulation markers
3.7.1 Monoclonal enzyme immunoassay for plasma F1+2 values
The incubation of Genta-P and Genta-L coated Alpha Graft® PTFE grafts in human blood caused the formation of a smaller degree of human prothrombin fragment F1+2 (250 pmol/l for Genta-P and 720 pmol/l for Genta-L) compared to uncoated PTFE prostheses (1,760 pmol). Genta-SDS coated Alpha Graft® PTFE grafts and coated SEALPTFE™ grafts showed similar increased F1+2 values at 2,880 and 2,610 pmol/l, respectively. The highest F1+2 values by far were assessed for InterGard® grafts at 7,980 pmol/l (Fig. 4). The range of citrated plasma for healthy adults averages from 69 to 229 pmol/l.
Coagulation marker prothrombin fragment F1+2 values in non anticoagulated human blood after 7 min of incubation for coated PTFE grafts (Alpha Graft®, Alpha research, Berlin, Germany), gelatine-sealed PTFE grafts (SEALPTFE™, Vascutek, Hamburg, Germany) and InterGard® grafts (InterVascular, La Ciotat, France)
3.8 Amidolytic substrate assay for factor XIIa-like activity
The assessment of factor XIIa-like activity in human blood showed that all tested grafts activated factor XIIa in the same range between 0.58 and 0.85 ng/ml as uncoated PTFE prostheses of about 0.81 ng/ml (Fig. 5).
3.9 Complement C3a-desArg ELISA
The quantitative assessment of C3a-desArg showed different results depending on the test graft. Gentamicin salt coated Alpha Graft® PTFE grafts and coated SEALPTFE™ grafts showed very low C3a-desArg concentrations of 140 ng/ml for Genta-L coatings, 155 ng/ml for coated SEALPTFE™ grafts, 194 ng/ml for Genta-P and 300 ng/ml for Genta-SDS coated Alpha Graft® PTFE grafts (compared to uncoated reference grafts at 364 ng/ml). The highest C3a-desArg values were assessed for InterGard® grafts at 594 ng/ml (Fig. 6).
4 Discussion
The use of synthetic vascular prostheses has tremendously changed vascular surgery, but post-operative infections give rise to an unexpected challenge for both surgeons and patients.
The infection risk for vascular grafts depends on the type of graft used. Allografts represent the most infection susceptible graft for different reasons: lack of protection through a vital endothelium, bacterial adherence on the implant during intra-operative contamination and receptor mediated attachment to the developed conditioning film after implantation result in a hardly avoidable intra-operative contamination. At the same time the immune defense is suppressed as a consequence of insufficient perfusion and capillarization. This is also the reason for the bad prospects of antibiotic treatment.
In general, bacterial infection of allografts is abetted through their structure and their initiated reactions inside the host. The porous architecture of ePTFE grafts or the fibrin network of pre-clotted, knitted Dacron® prostheses enables pathogens to immigrate into areas where the host’s immune cells can not follow. Through unspecific foreign body reactions, pathogens can be activated which promotes infection. The ability of pathogens to adhere to the implant surface depends for instance on the type and structure of bacteria, their ability to secrete mucin and the physical and chemical characteristics of the graft. Biofilm formation plays a crucial role in establishing a biomaterial-associated infection [11].
The most common way to prevent biofilm formation on medical devices is the incorporation of antibiotics/antiseptics into its surface material in order to kill incoming planktonic cells before they can adhere and start biofilm formation. An antibiotic-modified surface is therefore a helpful and established strategy. In this respect, aminoglycosides like gentamicin represent preferable antibiotics since they are effective against staphylococci and aerobic gram-negative bacilli and at the same time are considered to be safe for prophylactic therapy with a prolonged post-antibiotic effect. Gentamicin has already proven its effectiveness in vascular surgery by protecting gelatine-sealed grafts [12] and in eradicating microbial adherence of staphylococcal isolates from vascular grafts [13].
The present work is based on the application of gentamicin salts of palmitic acid, lauric acid and sodium dodecylsulfate for the development of anti-infective coatings of PTFE grafts. In order to characterize these newly developed anti-infective PTFE grafts a new methodology with regard to a systematic in vitro analysis initially had to be established, since data concerning a systematic in vitro analysis of vascular grafts with anti-infective properties are barely available. In particular, the impacts of anti-infective coatings on haemostasis and cytotoxicity have been neglected so far. The vast majority of published studies refer to insights from the clinical point of view. However, Matl et al. [14] presented a valuable systematic analysis for anti-infective coatings for PTFE grafts with gentamicin and teicoplanin. Contrary to the presented work, both drugs were incorporated into lipid-like carriers and there was no chemical binding between drug and carrier molecules. Generated coatings show well anti-infective effects of gentamicin coatings on vascular prostheses [14].
Results of mechanical stabilization tests showed that PTFE grafts with their porous structures are good carriers for fatty acid salts with excellent mechanical properties. Since it is possible to totally dissolve gentamicin fatty acid salts in an organic solvent, gentamicin was considered to be homogeneously distributed in the developed coatings. Therefore, the manufacturing process for coated PTFE grafts is considered to be reproducible.
The developed coatings ensured a continuous drug release over 24 h and therefore guarantee a high local antibiotic concentration around the graft right after implantation of the prosthesis. In comparison to a systemic gentamicin application by infusion, side effects for the host organism such as ototoxicity can thereby be reduced.
Experiments concerning the anti-infective efficiency of coated PTFE grafts revealed that the anti-bacterial protection was comparable to SEALPTFE™-prostheses, coated with Rifampicin and was even better compared to Dacron®-prostheses (InterGard®) coated with silver. Microbiological experiments gave evidence that an effective protection against high pathogen concentrations even with contamination of the graft after a 2 h preelution is build up as a consequence of high local antibiotic concentrations measured in the near environment of the coated prostheses.
In recent studies this type of gentamicin salt has already proven itself as an effective anti-infective coating material for commercially available Tilastan® layers of uncemented implants with respect to the inhibition of biofilm formation of S. aureus and S. epidermidis [15, 16].
The influence of coatings on the mechanism of haemostasis was investigated in detail. In thrombelastography investigations changes in coagulation parameters were assessed. These experiments have shown that especially the Genta-SDS-coatings showed a distinct prolongation of the CT accompanied by lower MCF. These observations were also made with respect to Genta-L, while Genta-P only showed a prolongation of the CT.
In order to characterize clot formation in the presence of gentamicin fatty acids in more detail, prothrombin fragment F1+2, the activated Factor XII and the thrombin-antithrombin III-complex were used as markers of the activated coagulation and measured by ELISA. Those markers activated by coated PTFE grafts showed unaltered concentration levels compared to uncoated PTFE grafts. Only Genta-SDS-coatings provoked an increase of these factors with appearance of haemolysis. SEALPTFE™ and InterGard® as well as palmitate-coatings showed the best haemocompatible properties. The behavior of palmitate and laurate-compounds concerning the complement system was neutral. Genta-SDS-coatings clearly activated the complement system resulting in an increase of the concentration of the complement factor C3a.
The complement system activation caused by Genta-SDS was also reflected in cytotoxicity studies performed with mouse fibroblasts. Experiments with incubated mouse fibroblasts indicate that pure palmitic acid and laurate-compounds showed little and functionally acceptable cytotoxic levels. Compared to the commercially available silver-acetate coated InterGard® graft, palmitate- and laurate-coatings showed a 13.6–22.8 % less cytotoxicity.
5 Conclusions
In this study we demonstrated the development of a drug coating system for ePTFE vascular grafts consisting of gentamicin fatty acid salts to be contemplated as an effective implant protection due to an effective local drug release system. Gentamicin palmitate as one of the three tested gentamicin salts was considered to be the most suitable coating for vascular prostheses with regard to anti-infective efficiency, bio- and haemocompatibility. Such coatings showed comparable results in view of commercially available SEALPTFE™ and knitted silver Dacron® and might be a suitable alternative to these clinically used vascular prostheses.
References
Saleem BR, Meerwaldt R, Tielliu IF, Verhoeven EL, van den Dungen JJ, Zeebregts CJ. Conservative treatment of vascular prosthetic graft infection is associated with high mortality. Am J Surg. 2010;200(1):47–52.
Twine CP, McLain AD. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev. 2010;5:CD001487.
Vollmar J, Buettner-Ristow A. Diagnostic and clinical aspects of septic complications in vascular surgery (author’s transl). Langenbecks Arch Chir. 1976;342:505–9.
Pounds LL, Montes-Walters M, Mayhall CG, Falk PS, Sanderson E, Hunter GC, et al. A changing pattern of infection after major vascular reconstructions. Vasc Endovascular Surg. 2005;39(6):511–7.
Bandyk DF, Novotney ML, Back MR, Johnson BL, Schmacht DC. Expanded application of in situ replacement for prosthetic graft infection. J Vasc Surg. 2001;34(3):411–9.
Kaebnick HW, Bandyk DF, Bergamini TW, Towne JB. The microbiology of explanted vascular prostheses. Surgery. 1987;102(4):756–62.
Lehnhardt FJ, Torsello G, Claeys LG, Pfeiffer M, Wachol-Drewek Z, Grundmann RT, et al. Systemic and local antibiotic prophylaxis in the prevention of prosthetic vascular graft infection: an experimental study. Eur J Vasc Endovasc Surg. 2002;23(2):127–33.
Turgut H, Sacar S, Kaleli I, Sacar M, Goksin I, Toprak S, et al. Systemic and local antibiotic prophylaxis in the prevention of Staphylococcus epidermidis graft infection. BMC Infect Dis. 2005;5:91.
Vogt S, Kühn KD, Gopp U, Schnabelrauch M. Resorbable antibiotic coatings for bone substitutes and implantable devices. Materialwiss Werkstofftech. 2005;36(12):814–9.
Uckay I, Pittet D, Vaudaux P, Sax H, Lew D, Waldvogel F. Foreign body infections due to Staphylococcus epidermidis. Ann Med. 2009;41(2):109–19.
Schwank S, Rajacic Z, Zimmerli W, Blaser J. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob Agents Chemother. 1998;42(4):895–8.
Ginalska G, Osinska M, Uryniak A, Urbanik-Sypniewska T, Belcarz A, Rzeski W, et al. Antibacterial activity of gentamicin-bonded gelatin-sealed polyethylene terephthalate vascular prostheses. Eur J Vasc Endovasc Surg. 2005;29(4):419–24.
Edmiston CE Jr, Goheen MP, Seabrook GR, Johnson CP, Lewis BD, Brown KR, et al. Impact of selective antimicrobial agents on staphylococcal adherence to biomedical devices. Am J Surg. 2006;192(3):344–54.
Matl FD, Obermeier A, Repmann S, Friess W, Stemberger A, Kuehn KD. New anti-infective coatings of medical implants. Antimicrob Agents Chemother. 2008;52(6):1957–63.
Kuehn KD. In vitro release of gentamicinpalmitate coating in uncemented titanium implants. IJNBM. 2010;3(1):94–106.
Kuehn KD, Bruenke J. Effectiveness of a novel gentamicinpalmitate coating on biofilm formation of Staphylococcus aureus and Staphylococcus epidermidis. IJNBM. 2010;3(1):107–17.
Acknowledgments
This work was partially financial supported by the Heraeus Medical GmbH (Werheim, Germany). We would like to thank V. Vatou (Institute for Medical Microbiology and Hygiene at the Klinikum rechts der Isar, TU München) for her great assistance in microbial testing. Furthermore, we acknowledge T. C. Hohnschildt for her contribution to this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obermeier, A., Matl, F.D., Schwabe, J. et al. Novel fatty acid gentamicin salts as slow-release drug carrier systems for anti-infective protection of vascular biomaterials. J Mater Sci: Mater Med 23, 1675–1683 (2012). https://doi.org/10.1007/s10856-012-4631-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4631-5