Abstract
This study evaluated protocols to eliminate acetone from dental adhesives and their effect on the kinetic of water sorption and percent of conversion of these adhesives. Experimental methacrylate-based adhesives with increasing hydrophilicity (R2, R3, R5) were used as reference materials. Primer-like solutions were prepared by addition of 50 wt% acetone. Acetone elimination was measured gravimetrically before and after: a spontaneous evaporation, an application of air-drying at room temperature or application of 40°C air-drying. Protocols were performed from 15 to 60 s. Specimens of adhesive/acetone mixtures were photo-activated and tested for degree of conversion, water sorption and solubility. Data were analyzed by ANOVA and Bonferroni’s tests (α = 0.05). Complete acetone elimination was never achieved, but it was significantly greater after the 40°C air-drying application. Higher acetone elimination was observed for the least hydrophilic adhesive. Longer periods for acetone evaporation and heated air-stream can optimize polymerization and reduce the water sorption/solubility of adhesive system models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The water-wet bonding technique has been considered a universal procedure for coupling resin composites to dentin via hybridization with etch-and-rinse adhesives. In contemporary dental adhesives, high concentrations of relatively hydrophilic methacrylate monomers (i.e. hydroxyethylmethacrylate (HEMA); 3,3′-dimethacryloyloxyethyl ester of 3,4,3′,4′biphenyltetracarboxylic acid (BPDM) are generally blended with relatively hydrophobic adhesive monomers (i.e. 2,2-bis(4-2-hydroxy-3-methacryloyloxyproproxyphenyl)propane (Bis-GMA) to enhance bonding to water-wet dentin [1–4]. To facilitate the mixing of hydrophilic with hydrophobic monomers and to avoid phase separation between these components, manufacturers have also added volatile solvents such as ethanol or acetone when formulating dental adhesives. Since these organic solvents exhibit higher vapor pressure than water, they are thought to be essential in facilitating the displacement of water from the acid-etched dentin matrix; ensuring better monomers infiltration into the micro- and nanoporosities left between the collagen fibrils and, thus, improving resin micro-retention to dentin [4].
The observation that resin-bonded dentin is not as well sealed as dentin covered with a smear layer raises concerns as to whether dental adhesives can ever truly seal dentin [5, 6]. Apparently, nanometer-sized spaces remain filled with water after attempts to evaporate solvents from resin-infiltrated dentin [7]. It is expected that during the hybridization of dentin with hydrophilic monomers, all solvent and water are completely eliminated from the collagen interfibrillar spaces in order to guarantee optimal monomer infiltration/conversion [3, 8–12]. It has been shown that among other factors, water sorption of adhesives and resin-dentin interfaces is dependent on the presence of residual solvent within these structures [13] and degree of hydrophilicity of adhesives [10, 12–14]. Thus, the presence of residual solvent, combined with the use of hydrophilic comonomers applied to wet dentin may synergistically compromise the requirements for perfect sealing and durable coupling between resin composites and resin-bonded dentin.
To permit correlations between resin comonomers’ hydrophilicity and the effect of residual solvent on adhesives’ performance, some studies have investigated experimental dental adhesives [11, 13–17] in which the hydrophilicity characteristics can be predicted by calculating the Hoy’s solubility parameters of each mixture. Hoy’s triple solubility parameters allow one to calculate the relative contribution of dispersive (δd), polar (δp) and hydrogen bonding (δh) forces to the total cohesive energy density (δt) of polar solvents [17–21]. Such an approach has been very useful to explain the interaction between solvent and dentinal collagen matrix in order to improve resin infiltration in bonding procedures to dentin [17, 19, 20]. Furthermore, as these parameters express the total cohesive energy of a material/substance, they can also be used to predict the eventual interaction between fluids, thereby helping one to understand the affinity between different solvents and resin comonomers [11, 17].
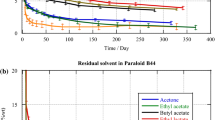
For instance, it was previously shown that these experimental adhesives solvated with 50 wt% acetone, 50 wt% ethanol, 50 wt% acetone/water or 50 wt% ethanol/water mixtures retained from 5 to 10% of the added solvent, even after blowing air for 120 s [11], a period up to 10–12 times longer than that recommended by the majority of dental adhesives manufacturers. Although there is a concern regarding the negative effects of the residual solvent on the performance of dental adhesives and their derived resin-dentin bonds [15], there has been little systematic effort to achieve maximum solvent evaporation during the hybridization of acid-etched dentin. While some manufacturers recommend application of a “gentle” air-drying (varying from 1 to 5 s), other manufacturers concerned about presenting more oxygen to the adhesive layer [16] do not recommend any procedure to facilitate the solvent evaporation.
The objective of this study was to evaluate the efficiency of different protocols in facilitating acetone evaporation from dental adhesives with different degree of hydrophilicity. Because it is not practical to investigate commercial adhesives of unknown quantitative composition, experimental comonomer blends with increasing degree of hydrophilicity (as determined by their Hoy’s solubility parameters) were used to produce different formulations that were then supplemented with a known amount of acetone. The following hypotheses were tested: (1) different protocols produce different solvent evaporation from the experimental adhesives; (2) the solvent evaporation alters the degree of conversion and water sorption and solubility of these adhesives and (3) the solvent evaporation depends on the hydrophilicity of the experimental adhesives.
2 Materials and methods
2.1 Adhesives preparation
Three experimental comonomer resin blends (R2, R3 and R5) were evaluated as potential dentin/enamel adhesive systems. These experimental resin blends were purposely formulated to be ranked in an increasing order of hydrophilicity (R2 < R3 < R5), based on their triple Hoy’s solubility parameters [17, 18], as listed in Table 1. Resin R5 is similar to one-step self-etch adhesives because it contain a comonomer with an acidic functional group that is a methacrylate derivative of phosphoric acid (i.e. Bis[2-(methacryloyloxy)ethyl] phosphate) [10, 18]. It is very hydrophilic when compared to resin R2, which consist of relatively more hydrophobic dimethacrylates. Resin R2, therefore, is similar to non-solvated bonding agents of three-step etch-and-rinse and two-step self-etch adhesive systems [18]. Resin R3 has an intermediary hydrophilicity and contains a typical composition of two-step etch-and-rinse adhesives [17]. These experimental resins were tested in the form of either neat or solvated adhesives that were mixed with pure acetone (Merck KgaA, Darmstadt, Germany) to produce primers, containing 50% comonomers/50% acetone (w/w%). Freshly prepared mixtures were ultra-sonicated for 5 min in hermetically sealed containers to ensure a single homogeneous phase.
2.2 Gravimetric measurements of adhesives before and after evaporation of solvent
Twenty micro liter-aliquots of each adhesive/acetone mixture were carefully and individually dispensed into 50-μl polypropylene screw-capped tubes and had their initial mass (m i ) measured using an analytical balance (Mettler Toledo Inc., Columbus, OH, USA). For one-third of these mixtures, the polypropylene tubes were then left opened so that the solvent evaporation could occur spontaneously. The weight of these specimens was repeatedly recorded after 15, 30 and 60 s. For the remaining two-thirds of the adhesive/acetone specimens, the solvent evaporation was facilitated by the application of air-drying either at room temperature (23°C) or at 40 ± 1°C. In both conditions, the air stream was blown by an adapted dental triple syringe, which was connected to a resistance-wiring device that permitted the air to be heated, when necessary. This adapted air-drying device was positioned 2 cm from the adhesives’ surface. All experiments were carried out at 23°C and under a relative humidity of 60%. Air-drying application (at room temperature or heated) was performed for three different periods of time: 15, 30 or 60 s with an air speed of 4.8 m/s and flow rate of 0.0124 m3/s. The final mass (m f ) of the adhesive/solvent mixtures was gravimetrically measured immediately after the air-drying application. Eight samples were prepared per adhesive per experimental condition (spontaneous evaporation, air-drying at room temperature and heat air-drying). Non-solvated versions of the experimental adhesives R2, R3 and R5 were submitted to the same experimental protocols in order to verify whether they can have their mass altered by loss of other components than acetone.
The effect of experimental protocols on facilitating acetone evaporation from adhesives was expressed in terms of the percentage of adhesives mass loss (LM) using the following equation:
where m i and m f are, respectively, the mass of the sample before and after the solvent evaporation.
Percentages of adhesives mass loss were analyzed by three-way ANOVA and Bonferroni tests, having the following main factors: type of resin blend (R2, R3 or R5), protocol for solvent evaporation (spontaneous, air-drying at room temperature and heat air-drying) and time of solvent evaporation (15, 30 or 50 s). Statistical significance was preset at α = 0.05.
2.3 Resin disk preparation
Resin disks of each experimental adhesive (neat or solvated) were produced in a brass mold (3.0 mm diameter, 0.5 mm thick). Thus, 20 µl of the liquid adhesive was directly dispensed to completely fill the mold. Solvent evaporation was performed according to the protocols previously described. At the end of the specified evaporation time, a glass cover slip was placed on the top of the experimental adhesives to exclude atmospheric oxygen and prevent additional acetone evaporation. Photo-activation was immediately performed using a quartz-tungsten-halogen-light source at delivered 650 mW/cm2 for 30 s (Elipar TriLight, ESPE, Germany). After removal from the mold, the bottom of the resin disks was further photo-cured for another 30 s. Selection of curing time was determined in a pilot experiment by measuring a baseline microhardness of the surface of the resin disks (unpublished data). With the total curing time of 60 s, the resins exhibited a mean Knoop hardness of 13 ± 4 KHN (HMV-2, Shimadzu, Tokyo, Japan). Ten specimens were produced with each experimental neat and solvated adhesive. These specimens were randomly assigned into two groups (n = 5 per group) to evaluate the degree of conversion and the water sorption and solubility after storage in water.
2.4 Degree of conversion analysis
Following storage in a desiccator to obtain a constant dry mass, the adhesive disks were pulverized into fine powder using an agate mortar and pestle. Adhesives powder was mixed with infrared grade potassium bromide (KBr) powder at a ratio of 3:180 mg [19]. Five KBr pellets were obtained from each of tested cured resins. Infrared-spectra of KBr/resin pellets were collected in transmission mode using a Fourier transform infrared spectroscopy (FTIR Shimadzu 8300, Shimadzu, Tokyo, Japan) equipped with a KBr beam splitter and a mercury cadmium telluride detector. A blank KBr pellet was used for the collection of the background spectrum. For each specimen, multiple spectra were collected in the range of 4000~650 cm−1 at a resolution of 4 cm−1. FTIR-spectra of uncured adhesives were also obtained as reference for calculation of the degree of conversion (DC). From the absorbance of uncured adhesives, a calibration curve was generated allowing for correlation of (C=C) absorption ratios with known molar concentration ratios. The degree of conversion was calculated from the equivalent aliphatic (absorbance peak located at 1638 cm−1)/aromatic (absorbance peak located at 1608 cm−1) molar ratios of cured (C) and uncured (U) specimens [20]. Percentage of degree of conversion (%DC) of all neat and solvated resin blends was estimated based on the formula:
Degree of conversion for neat and solvated resins was analyzed by a two-way ANOVA with the protocol for solvent evaporation (spontaneous, air-drying at room temperature and heat air-drying) and the time of solvent evaporation (15, 30 or 60 s) as the main factors. Post hoc multiple comparisons were performed using Bonferroni test. Statistical significance was preset at α = 0.05.
2.5 Water sorption and solubility
Water sorption and solubility were determined using the following modifications of ISO 4049: specimen dimensions (3.0 mm in diameter, instead of 15 mm) and different periods of water gain/loss measurements. After preparation, the resin disks were all pre-dried in a sealed desiccator containing fresh silica gel (at 37°C) and repeatedly weighed at 24 h intervals, until a constant mass (m1) was obtained (i.e. variation lower than 0.02 mg in 24 h). They were individually immersed in deionized water at 37°C. At fixed time intervals, the specimens were removed from the vials, washed in running water for 5 s, blot-dried, weighed and returned to water. Several readings were taken during the first day (i.e. every 30 min for 12 h) and then 12 h after this last reading. For all materials, equilibrium of specimen mass was attained between the 12th and 36th hof storage in water. At this time the resin disks were washed in running water, gently wiped with absorbent paper, and weighed in an analytical balance for m 2 determination. The resin disks were re-dried in a desiccator, as previously described, and weighed daily until a constant mass (m 3) was re-obtained. Water sorption (WS) and solubility (SL) were calculated using the following formulae [21]:
where V is the volume of each resin disk (in mm3).
Means of water sorption and solubility were analyzed by two individual multiple-way ANOVA (one for water sorption and other for solubility data), having as main factors: the type of resin blend (R2, R3 or R5), the type of solvent (ethanol or acetone), the protocol of solvent’s evaporation (spontaneous, air-drying at room temperature and heat air-drying) and the time of solvent’s evaporation (15, 30 or 50 s) as main factors. Post hoc multiple comparisons were performed using Bonferroni test. Statistical significance was preset at α = 0.05.
3 Results
3.1 Adhesives’ mass loss
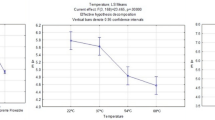
Non-solvated adhesives did not exhibit significant change of their mass either by letting them evaporate spontaneously (p > 0.05) or by directing moving air streams (heated or at room temperature) on them (p > 0.05) (data not shown). Means of loss of mass (in percentage) for solvated adhesives are summarized in Table 2. None of the treatments was able to completely eliminate the solvent that was incorporated into the experimental adhesives. Loss of mass of solvated adhesives submitted to spontaneous evaporation was minimum (i.e. ranging between 1 and 4%) even after 60 s. Differences in the loss of mass between solvated adhesives R2 and R3 and between R3 and R5 were not significant when the solvent were allowed to evaporate spontaneously (p > 0.05). For all experimental adhesives, the highest percentage of mass loss, meaning higher degree of solvent evaporation, was observed after the application of heated air to the disks for 60 s. Under this condition there is no significant difference between adhesives (p > 0.05). Nevertheless, with the application of room temperature or 40°C air streams, the least hydrophilic adhesive (R2) exhibited the highest percentage of solvent evaporation compared with the most hydrophilic adhesive (R5) (p < 0.05). In general, the longer the period for solvent evaporation, the higher was the mass loss, regardless of the intrinsic hydrophilicity of adhesives.
3.2 Degree of conversion
Means of degree of conversion for non-solvated and solvated experimental adhesives are summarized in Table 3. There is no difference in the degree of conversion for non-solvated adhesives, regardless of the treatment and hydrophilicity of adhesives (p > 0.05). For solvated adhesives, it an increase in degree of conversion was seen with an increase in evaporation time (60 > 30 > 15 s) with heated air-drying > room temperature air-drying > spontaneous evaporation. In general, when the solvent was evaporated spontaneously or by room temperature air, the most hydrophilic adhesive (R5) exhibited higher degree of conversion than the least hydrophilic adhesive (R2) (p < 0.05), at the same fixed period of time. Conversely, at any given evaporation time, no difference in the degree of conversion was observed between hydrophilic and hydrophobic adhesives when the solvent was evaporated by 40°C air (p > 0.05).
3.3 Water sorption and solubility
Results of water sorption and solubility for non-solvated and solvated experimental adhesives are summarized in Tables 4 and 5. There was no change in water sorption values for non-solvated adhesives, regardless of the evaporation treatment of adhesives (p > 0.05). However, there was an increase in water sorption in the non-solvated adhesives with increases in resin hydrophilicity with R5 > R3 > R2. Generally, the non-solvated adhesives exhibited values of water sorption that were significantly lower when compared to their correspondent acetone-solvated versions (p < 0.05). For acetone-solvated adhesives, there was a decrease in water sorption that varied with time (60 > 30 > 15 s) and drying protocol for solvent evaporation (40°C air-drying > room temperature air-drying > spontaneous evaporation). Overall, the most hydrophilic adhesive (R5) exhibited the highest values of water sorption in comparison with the least hydrophilic adhesives (R2 and R3) (p < 0.05), regardless of the time and protocol for solvent evaporation (Table 4).
Following a similar trend, there was no change in solubility values for non-solvated adhesives, regardless of the drying treatment and hydrophilicity of adhesives (p > 0.05). In general, the non-solvated adhesives exhibited values of solubility that were significantly lower when compared to their correspondent acetone-solvated versions (p < 0.05). For acetone-solvated adhesives, a decrease in solubility values, varying with the evaporation time (60 > 30 > 15 s) and protocol for solvent evaporation (heat air-drying > room temperature air-drying > spontaneous evaporation) was observed. As a general rule, the most hydrophilic adhesive (R5) exhibited the highest values of solubility in comparison with the least hydrophilic adhesives (R2 and R3) (p < 0.05), regardless of the time and protocol for solvent evaporation (Table 5).
There was no significant correlation between percent conversion and the water sorption/solubility of any of the resins (data not shown).
4 Discussion
The results of this study revealed that non-solvated adhesives did not exhibit significant change in mass after air-drying (40°C or at room temperature) for any tested period. In addition none of these treatments affected either the degree of conversion or the water sorption/solubility of these non-solvated adhesives. Conversely, for acetone-solvated adhesives, the same properties (i.e. degree of conversion and the water sorption/solubility) were substantially enhanced by the evaporation time and air-drying protocol for solvent evaporation. These results indicate that the percent conversion of dental adhesives can be significantly increased after a consistent removal of their compositional solvent. This requires the acceptance of the first and second hypotheses of this study. The hydrophilicity of experimental adhesives significantly influenced the evaporation of solvent for the majority of tested conditions, with the most hydrophilic adhesive allowing the least amount of acetone evaporation. Nonetheless, after the application of heated air-drying for 60 s, all acetone-solvated adhesives exhibited the same percentage of loss of mass, indicating that the hydrophilic and hydrophobic resin comonomers permitted the amount of acetone evaporation. Thus, these results induce the partial acceptance of the third hypothesis of this study.
Previous reports have already shown that excess residual solvent is detrimental for monomers conversion [13, 22–24] as it produces polymers that are more prone to absorb water, leach components and degrade over time [11, 14]. The results of the current study confirms that removal of residual acetone increases the quality of adhesives by forming a structure which is more cohesive, densely-packed [23, 25] and less permeable [26, 27], all characteristics that are required to seal dentin against outward fluid leakage. Additionally, incomplete solvent evaporation has been considered as one of the most serious source of errors during the bonding procedure [28]. Residual solvent within comonomers probably hampers their interaction with the dental substrate and it also damages the propagation and growth of polymer network [27]. Current protocols for solvent evaporation recommended by dental adhesives’ manufacturers are, therefore, deficient and probably cause adhesives to under polymerize.
Complete solvent/water evaporation has shown to be clinically problematic [29–31] especially when using the evaporation times recommended by manufacturers [32, 33]. When solvated-adhesives are dispensed into a container, like in the present study, the rate of solvent evaporation is basically governed by factors such as the vapor pressure of mixture (i.e. solvent + monomers), the environmental temperature/relative humidity and the air-exposed free surface of mixture. Even so, when solvated-adhesives are infiltrated into demineralized dentin they normally result in a thin film (≈30 μm), wherein there are other interacting factors that may modify the final result [34]. For instance, the level of interaction between monomers and/or solvents with collagen or non-collagenous proteins of dentin matrix may change the rate of solvent evaporation/retention, thereby influencing the clinical procedure [35, 36]. Despite the current results cannot be fully extrapolated to clinical situation because the effects of acetone evaporation was not evaluated while it was mixed with water as it is during the procedures to wet dentin, other studies have demonstrated the benefits of using a heat air-stream to optimize the quality of resin-dentin bonds, such as a significant reduction of the silver nitrate uptake expression within dentin-adhesive interfaces created with commercial adhesives [37, 38]. Such improvement in the quality of resin-dentin bonds was claimed to occur in virtue of a better solvent evaporation capacity, associated with a consequent better packing-density of the polymer network achieved when using heated air-stream.
Solvent retention in dental adhesives may also depend on its affinity to other components of the mixture, such as resin comonomers and photo-initiators [35]. Similarly to the present study, Yiu et al. [11] also concluded that the percentage of retained solvent in experimental adhesives was significantly influenced by the hydrophilicity of resin comonomers, with the most hydrophilic adhesive exhibiting the highest percentage of residual solvent. The present results clearly showed that, for the majority of the tested conditions, the most hydrophilic solvated adhesive (R5; Table 1) exhibited the highest amount of residual acetone (Table 2) and, as well, the lowest degree of conversion (Table 3) combined with the highest values of water sorption/solubility (Tables 4, 5).
Interestingly, when a heated air-stream was applied for 60 s, the difference in adhesives’ hydrophilicity was no longer a significant determinant of solvent evaporation, with all tested solvated adhesives showed the same percentage of loss of mass (Table 2). These results suggest that increasing the temperature of air-drying and prolonging the duration of such application may have likely increased the kinetic energy of the molecules in the adhesive system promoting the increase of molecular vibration and facilitating the breakdown of intermolecular bonds between solvent and polar groups of resin comonomers, thus favoring solvent evaporation. Elevations in temperature also increase the vapor pressure of solvents.
Solvated adhesives that were exposed to 40°C air for 60 s exhibited the same degree of conversion regardless of their hydrophilicity. Previously, higher degrees of solvent evaporation were found even in most hydrophilic adhesive, when air-drying temperature was raised to 40°C (60 s), almost the same temperature employed in previous study [33]. Unlike the results of Nunes et al. [33], we found higher solvent evaporation rate (about 30% evaporated) when the drying air temperature was raised for HEMA-containing resins, compared to room temperature air-drying (about 15% evaporated). For this group, there was a clear relation between the removal of residual acetone and degree of adhesive conversion. Resins with higher monomer conversion exhibited lower water sorption and solubility compared with those of solvated adhesives that only evaporated spontaneously or with air-drying at room temperature. The increase in temperature is one of the mechanisms that decrease the adhesive viscosity during the early stage of the adhesive photo-curing period [24]. Also, the presence of lower concentrations of acetone reduced the viscosity of the resins in a level that the intermolecular spacing became greater in its free volume, providing more mobility of the monomer chain and greater rate of diffusion of radicals due the probability of chain collision [23, 29]. So, air-drying at 40 (±1)°C as well as the lower residual solvent may decreases the comonomer viscosity resulting in improved conversion of the monomers into polymers.
The presence of residual solvent notwithstanding, the degree of water sorption/solubility were strongly correlated with the hydrophilicity of adhesives subjected to spontaneous evaporation or an air stream at room temperature, which is in agreement with results of previous studies [10–14]. That is, the more hydrophilic the adhesive, the greater the water sorption and the more the polymer network is plasticized by absorbed water [39]. This causes polymer swelling that reduces the frictional forces between adjacent polymer chains [40]. At a high level of absorbed water, polymer chains can undergo a relaxation process, thereby facilitating the elution of unreacted monomers and/or solvents trapped in the polymer network [40]. More hydrophilic polymers have superior capacity to absorb water due to the presence of higher amount of polar domains [10, 12, 39] that hydrogen bond with water and, at the same time, they also have a superior capacity of relaxation, which may permit faster elution of unreacted monomers/solvents compared with more hydrophobic polymers [40, 41]. In the long-term, the permanent solubility of hydrophilic resin blends and the replacement of those unpolymerized monomers by water may be the cause of hydrolytic breakdown of resin compounds, instead of a simple release of unreacted and/or pendent monomers.
There is certainly need for more accurate research to determine the effects of long-term release from resin-based dental materials, in special regarding those with a higher hydrophilic characteristic that could be, in thesis, more prone to deteriorate over time. The local and systemic toxic effects of dental adhesives by-products/unreacted monomers are still neglected. Studies with methacrylated-based dental resins point out that these materials are highly prone to hydrolytic salivary degradation [42] and they contain several ingredients which were shown to be cytotoxic, genotoxic and mutagenic [43]. Based on these concerns, the present results suggest that the current regimes indicated for solvent evaporation from dental adhesives should be modified in order to improve the percent of conversion and minimize the solubility of these materials.
5 Conclusion
In conclusion it can be consider that longer solvent evaporation regimes (i.e. 60 s) and the use of 40°C air-stream were the most effective maneuvers in evaporating acetone and this optimized the degree of conversion and reduced the solubility/water sorption tendency of etch-and-rinse one-step adhesive models.
References
Kanca J. Resin bonding to wet substrate. I-Bonding to dentin. Quintessence Int. 1992;23:39–41.
Kanca J. Improved bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123:35–43.
Jacobsen T, Söderholm KJ. Some effects of water on dentin bonding. Dent Mater. 1995;11:132–6.
Jacobsen T, Söderholm KJ. Effect of primer solvent, primer agitation, and dentin dryness on shear bond strength to dentin. Am J Dent. 1998;11:225–8.
Bouillaguet S, Duroux B, Ciucchi B, Sano H. Ability of adhesive systems to seal dentin surfaces an in vitro study. J Adhes Dent. 2000;2:201–8.
Carrilho MR, Tay FR, Sword J, Donnelly AM, Agee KA, Nishitani Y, Sadek FT, Carvalho RM, Pashley DH. Dentine sealing provided by smear layer/smear plugs versus adhesive resins/resin tags. Eur J Oral Sci. 2007;115:321–9.
Hashimoto M, Tay FR, Ito S, Sano H, Kaga M, Pashley DH. Diffusion-induced water movement within resin-dentin bonds during bonding. J Biomed Mater Res B. 2006;79B:453–8.
Paul SJ, Leach M, Rueggeberg FA, Pashley DH. Effect of water content on the physical properties of model dentine primer and bonding resins. J Dent. 1999;27:209–14.
Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002;81:472–6.
Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg FA, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–59.
Yiu CK, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005;26:6863–72.
Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, Yiu CK, Carrilho MR. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–80.
Malacarne-Zanon J, Pashley DH, Agee KA, Foulger S, Alves MC, Breschi L, Cadenaro M, Garcia FP, Carrilho MR. Effects of ethanol addition on the water sorption/solubility and percent conversion of comonomers in model dental adhesives. Dent Mater. 2009;25:1275–84.
Yiu CK, King NM, Carrilho MR, Sauro S, Rueggeberg FA, Prati C, Carvalho RM, Pashley DH, Tay FR. Effect of resin hydrophilicity and temperature on water sorption of dental adhesive resins. Biomaterials. 2006;27:1695–703.
Carvalho RM, Mendonca JS, Santiago SL, Siliveira RR, Garcia FCP, Tay FR, Pashley DH. Effect of HEMA/solvent combinations on bond strength to dentin. J Dent Res. 2003;82:597–601.
Yiu CK, King NM, Pashley DH, Suh BI, Carvalho RM, Carrilho MR, Tay FR. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25:5789–96.
Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, García-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–20.
Barton AFM. Handbook of solubility parameters, other cohesion parameters. 2nd ed. Boca Raton: CRC Press; 1991. p. 98–103.
Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–9.
Ruyter IE, Svendsen SA. Remaining methacrylate groups in composite restorative materials. Acta Odontol Scand. 1978;36:75–82.
Andrzejewska E. Photopolymerization kinetics of multifunctional monomers. Prog Polym Sci. 2001;26:605–65.
Pashley DH, Agee KA, Carvalho RM, Lee KW, Tay FR, Callison TE. Effects of water and water-free polar solvents on the tensile properties of demineralized dentin. Dent Mater. 2003;19:347–52.
Elliott JE, Lovell LG, Bowman CN. Primary cyclization in the polymerization of bis-GMA and TEGDMA: a modeling approach to understanding the cure of dental resins. Dent Mater. 2001;17:221–9.
Nunes TG, Ceballos L, Osorio R, Toledano M. Spatially resolved photopolymerization kinetics and oxygen inhibition in dental adhesives. Biomaterials. 2005;26:1809–17.
Holmes RG, Rueggeberg FA, Callan RS, Caughman F, Chan DC, Pashley DH, Looney SW. Effect of solvent type and content on monomer conversion of a model resin system as a thin film. Dent Mater. 2007;23:1506–12.
Ye Q, Spencer P, Wang W, Misra A. Relationship of solvent to the polymerization process, properties and structure in model adhesives. J Biomed Mater Res A. 2007;80:342–50.
Ajithkumar S, Patel NK, Kansara SS. Sorption and diffusion of organic solvents through interpenetrating polymer networks (IPNs) based on polyurethane and unsaturated polyester. Eur Polym J. 2000;36:2387–93.
Hashimoto M, Tay FR, Svizero NR, De Gee A, Feilzer AJ, Sano H, Kaga M, Pashley DH. The effects of common errors on sealing ability of total etch adhesives. Dent Mater. 2006;22:560–8.
Pashley EL, Zhang Y, Lockwood PE, Rueggeberg FA, Pashley DF. Effects of HEMA on water evaporation from water-HEMA mixtures. Dent Mater. 1998;14:6–10.
Cho BH, Dickens SH. “Effects of the acetone content of single solution dentin bonding agents on the adhesive layer thickness and the microtensile bond strength”. Dent Mater. 2004;20:107–15.
Cadenaro M, Breschi L, Rueggeberg F, Suchko M, Grodin E, Agee K, Agee K, Di Lenarda R, Tay FR, Pashley DH. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent Mater. 2009;25:621–8.
Ikeda T, De Munck J, Shirai K, Hikita K, Inoue S, Sano H, Lambrechts P, Van Meerbeek B. Effect of evaporation of primer components on ultimate tensile strengths of primer-adhesive mixture. Dent Mater. 2005;21:1051–8.
Nunes TG, Garcia FC, Osorio R, Carvalho R, Toledano M. Polymerization efficacy of simplified adhesive systems studied by NMR and MRI techniques. Dent Mater. 2006;22:963–72.
Garcia G, Fernandes KB, Garcia FC, D’Alpino PH, da Rocha Svizero N, Wang L. Solvent retention of contemporary commercial dentin bonding agents in a demineralized dentin matrix. Eur J Dent. 2010;4:293–7.
Eddleston CL, Hindle AR, Agee KA, Carvalho RM, Tay FR, Rueggeberg FA, Pashley DH. Dimensional changes in acid demineralized dentin matrices following the use of HEMA-water versus HEMA-alcohol primers. J Biomed Mater Res A. 2003;67:900–7.
Pashley DH, Agee KA, Nakajima M, Tay FR, Carvalho RM, Terada RS, Harmon FJ, Lee WK, Rueggeberg FA. Solvent-induced dimensional changes in EDTA-demineralized dentin matrix. J Biomed Mater Res. 2001;56:273–81.
Klein-Júnior CA, Zander-Grande C, Amaral R, Stanislawczuk R, Garcia EJ, Baumhardt-Neto R, Meier MM, Loguercio AD, Reis A. Evaporating solvents with a warm air-stream: effects on adhesive layer properties and resin-dentin bond strengths. J Dent. 2008;36:618–25.
Reis A, Klein-Júnior CA, de Souza FH, Stanislawczuk R, Loguercio AD. The use of warm air stream for solvent evaporation: effects on the durability of resin-dentin bonds. Oper Dent. 2010;35:29–36.
Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–22.
Brazel CS, Peppas NA. Dimensionless analysis of swelling of hydrophilic glassy polymers with subsequent drug release from relaxing structures. Biomaterials. 1999;20:721–32.
Brazel CS, Peppas NA. Mechanisms of solute and drug transport in relaxing, swellable, hydrophilic glassy polymers. Polymer. 1999;40:3383–98.
Atkinson JC, Diamond F, Eichmiller F, Selwitz R, Jones G. Stability of bisphenol A triethylene-glycol dimethacrylate, and bisphenol A dimethacrylate in whole saliva. Dent Mater. 2002;18:128–35.
Van Landuyt KL, Nawrot T, Geebelen B, De Munck J, Snauwaert J, Yoshihara K, Scheers H, Godderis L, Hoet P, Van Meerbeek B. How much do resin-based dental materials release? A meta-analytical approach. Dent Mater. 2011;27:723–47.
Acknowledgments
This study was performed by Michele Bail as partial fulfillment of her M.S. degree at the University of Campinas. The authors gratefully acknowledge the technical support given by Mr. Marcos Blanco Cangiani and the editorial assistance of Ms. Michelle Barnes. The experimental resins used in this study were developed and donated by Bisco, Inc. This study was supported in part by Grants from CAPES, Brazil (P.I. Michele Bail); CNPq #306100/2010-0, FAPESP 07/54618-4 (P.I. Marcela Carrilho) and NIDCR # R01-DE-015306 (P.I. David Pashley).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bail, M., Malacarne-Zanon, J., Silva, S.M.A. et al. Effect of air-drying on the solvent evaporation, degree of conversion and water sorption/solubility of dental adhesive models. J Mater Sci: Mater Med 23, 629–638 (2012). https://doi.org/10.1007/s10856-011-4541-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4541-y