Abstract
Bioactive glasses have been developed as scaffolds for bone tissue engineering but combination with reindeer bone protein extract has not been evaluated. We investigated the effects of bone protein extract implants (5–40 mg dosages) with bioglass (BG) carrier on the healing of rat femur defects. Bioglass implants and untreated defects served as controls. All doses of extract increased bone formation compared with the control groups, and bone union was enhanced with doses of 10 mg or more. In comparison with untreated defect, mean cross-sectional bone area at the defect site was greater when implants with BG + 15 mg of extract or bioglass alone were used, bone density at the defect site was higher in all bioglass groups with and without bone extract, and the BG + 15 mg extract dosage marginally increased bone torsional stiffness in mechanical testing. Bioglass performed well as a carrier candidate for reindeer bone protein extract.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bone morphogenetic proteins (BMPs) are important growth factors in bone and cartilage regeneration [1, 2]. Despite modern surgical techniques, segmental long bone defects are often difficult to manage. Thus, the efficacy of bone protein extracts (containing bone morphogenetic proteins [BMPs]) with carrier materials in healing of long bone defects have been studied experimentally using segmental long bone defects in animal models with close similarity to clinical situations.
An optimal carrier matrix should fulfil several criteria. First, the matrix should be biocompatible and protect bone proteins from non-specific lysis. The ideal matrix should also be bioabsorbant, malleable, and withstand sterilization [3–6]. The carrier matrix should bond to the host bone without the formation of scar tissue, and resorb at the same rate as the bone is regenerated [7, 8]. Inorganic matrices have the advantage of being structurally strong, immunologically inert, osteoconductive, and biodegradable to various degrees [3, 4]. Bioactive glasses are inorganic materials that have been used in granular form to fill bone defects. They meet many criteria of optimal carrier matrices including biocompatibility and osteoconductivity, and are biodegradable. Thus, they are called surface-bioactive ceramics [9–13]. Bioactive glasses have been used as carriers of BMP, especially recombinant human BMP (rhBMP) [14–17]. In some studies, they have also been used as a carrier of native bone matrix [18, 19].
In the present study, we investigated the effect of reindeer bone protein extract combined with bioglass carrier on the healing of critical-sized bone defects in rat femurs. Our hypothesis was that bioglass could act as a carrier for reindeer bone protein extract and that this combination would heal the bone trauma in 8 weeks. The aim was to evaluate the healing effects of different doses of reindeer bone protein extract combined with bioglass carrier evaluated using native radiology, quantitative peripheral computer tomography (pQCT), and mechanical torsion tests.
2 Materials and methods
2.1 Bone protein extract
Native reindeer (Rangifer tarandus) protein extract was prepared from diaphyseal bone of the reindeer. Cortical bones from each animal were chilled immediately after death. The epiphyseal ends, bone marrow, and periosteum were mechanically removed and, after freezing in liquid nitrogen, the cleaned cortical bones were ground to a particle size of 1.0 mm3. The pulverized bone was demineralized in 0.6 M HCl and extracted in 4 M guanidine hydrochloride (GuHCl) at <9°C. The GuHCl-extracted solution was filtered through a tangential flow system and concentrated. The concentrated solution was dialyzed against deionised water and the water-insoluble material was collected. After re-dissolving in 4 M GuHCl solution, the water-insoluble material was dialyzed against 0.25 M citrate buffer, pH 3.1. The citrate-buffer-insoluble material was washed with deionised water and deep-frozen [20].
2.2 Reconstitution of implants
A dose of 5, 10, 15, or 40 mg of reindeer bone extract (BBS Ltd, Finland) was added to carboxymethyl cellulose (CMC, Sigma-Aldrich) to obtain a 3.2% (water/weight, w/w) gel. Bioglass granules (S53P4, size 500–800 μm, Vivoxid, Turku, Finland) were combined with extract-CMC-gel, and the mixture was shaped into a rod (diameter 5 mm) and lyophilized. Before drying, one 8 mm implant included 40% w/w of CMC-gel with a propriety amount of bone extract, and 60% (w/w) bioglass granules. The amount of CMC in the dried product was 2% (w/w). The doses of bone extract were chosen according to our previous studies [21–26]. Control implants were constructed in an identical fashion, but contained only bioglass carrier.
2.3 Animals and study groups
Fifty 2.5-month-old male Sprague–Dawley rats were used. Each animal was randomised to one of four groups for unilateral treatment with an implant: (1) bioglass (BG) + 5 mg bone protein extract (BG + 5 mg extract group); (2) bioglass + 10 mg bone protein extract (BG + 10 mg extract group); (3) bioglass + 15 mg bone protein extract (BG + 15 mg extract group); (4) bioglass + 40 mg bone protein extract (BG + 40 mg extract group); (5) bioglass alone (bioglass (BG) group); and (6) no implant (untreated group). Ten rats died within 24 h of the operation of no obvious cause. Thus, 40 of 50 rats survived to the end of the study (9 rats in the 40 mg extract group, and 6–7 rats in each of the other groups).
The study protocol was approved by the institutional animal experiment and ethical committee.
2.4 Surgical procedure
Surgery was performed under general anaesthesia with a blend of fentanylcitrate 80 μg/kg–fluanisone 2.5 mg/kg (Hypnorm®, Janssen Pharmaceutica, Inc., Beerse, Belgium) and midazolam 1.25 mg/kg (Dormicum®, Roche, Basel, Switzerland). Preoperatively, the animals were administered cefuroksim 20 mg/kg (Zinacef®, GlaxoSmithKline Manufacturing S.p.A., Verona, Italy) subcutaneously. A transverse skin incision was made over the posterior aspect of the thigh after shaving the hair around the left hind limb. The muscles were elevated circumferentially from the femoral diaphysis. A 25 mm × 4 mm × 2.5 mm polyacetyl plaster plate (POM, Vink Finland Ltd) was placed on the surface of the femur. The plate was temporarily secured in place with a stainless steel holder, which was exclusively designed and manufactured for this operation (Technical Services Unit, University of Oulu). The plate was fixed using 0.8-mm threaded Kirschner-wires, which have a 5-mm long threaded distal end (Synthes Oy, Helsinki, Finland). The canal for the threaded K-wire was predrilled through the plate and through both cortices of the femur using a 0.7 mm drill (Dremel 400, The Netherlands). The threaded K-wire was bent so as to easily screw the threaded end of the wire through the plate and into the bone. The correct depth for inserting the wire was determined by carefully dissecting the other side of the bone with a probe to confirm that the wire was penetrating the distal cortex of the bone. A total of 6 threaded K-wires were used for each plate, three on each sides of the bone defect, each screwed through two cortices. The excess wire was removed to the level of the plate using side-cutting pliers.
An 8-mm diaphyseal critical-sized defect was created using a stainless steel mini bur. After a thorough wash with saline, the implant was applied to the defect, or the defect was left empty for controls. The muscles and skin were closed in two layers using absorbable 4.0 sutures (Dexon®, Covidien, Mansfield, MA, USA). The pain medication after the operation consisted of buprenorfin (Temgesic®, Reckitt & Colman Pharmaceuticals, Inc, Richmond, England) at 0.01–0.05 mg/kg administered subcutaneously. In case of respiratory problems during anaesthesia, animals were treated with 1 mg of furosemid (Furesis® Orion, Espoo, Finland) subcutaneously. Eye gel (Viscotears®, Novartis Healthcare, Kobenhavn, Denmark) was applied to avoid eye dehydration during anaesthesia. The animals were allowed full activity in their cages postoperatively.
The rats were killed in a carbon dioxide (CO2) chamber after 8 weeks (untreated, bioglass alone, or 5, 10, and 15-mg extract groups), or after 10 weeks (40 mg extract group). The left leg of each rat was dissected from the body, wrapped in a saline wetted serviette, and frozen at −20°C until biomechanical analysis.
2.5 Radiographic evaluation of bone formation
Radiographs of the left femur (26 kV, 9.00 mAs, 0.09 s/exp, Mamex dc® ami, Orion Ltd., Soredex) were taken 3, 6, and 8 weeks postoperatively, and also 10 weeks postoperatively in the BG + 40 mg extract group. The radiographs were taken under neuroleptic analgesia (Hypnorm-Dormicum, 0.15–0.3 ml/100 g). Osiris 4.19 (Digital Imaging Unit, Geneva) software was used in the analysis of the radiographs. The percentage of orthopic new bone formation (BF) and the development of a bone union (BU) or non-union was estimated as described previously [26] according to the scoring system developed by Sciadini et al. [27].
2.6 Computed tomography
After the soft tissue on the frozen legs thawed it was removed from the bone and all left femurs were scanned using a pQCT device (Stratec XCT 960A, 5.21 software version, Norland Stratec Medizintechnik GmbH, Birkenfeld, Germany). A voxel size of 0.148 mm × 0.148 mm × 1 mm was used. One cross-sectional slice from the middle of the defect in each sample was scanned in two directions: first with a fixation system above the bone, then turned 90° axially. Average values of the two scans were used in the statistical analysis. Cross-sectional bone areas (mm2) and bone densities (mg/cm3) from the scanned slices of the samples were recorded using the pQCT software with a threshold of 169 mg/cm3 to distinguish the bone from the surrounding soft tissue, and a threshold of 464 mg/cm3 for the inner surface of the bone.
2.7 Mechanical tests
After pQCT imaging, the fixation system was removed from the samples. The bone ends were embedded into the moulds of the sleeves with dental stone (GC Fujirock, Improved Dental Stone, G-C Dental Industrial Corp., Tokyo, Japan). The torsional shaft was adjusted to 2 cm. After the cast hardened, the samples were placed in the torque machine [28] and torsionally loaded at a constant angular speed of 6°/s until failure. Maximum breaking load (Nm) and torsional stiffness (Nm/º) were recorded [29]. Mechanically unstable bones were not tested, and their values were considered to be zero in the statistical analysis.
2.8 Statistics
Statistical analysis was performed using SPSS for Windows ver. 15.0 statistical package (SPSS Inc., Chicago, IL, USA). The non-parametric Kruskall–Wallis test was used to evaluate the statistical differences between the groups. The Mann–Whitney test was used for pairwise comparisons between the treatment groups and the control groups (BG and untreated). The Benjamini–Hochberg procedure was used to correct P values for multiple comparisons [30]. Values of P < 0.05 were considered statistically significant. Results of pQCT are reported as the mean ± standard deviation, and results of mechanical tests are given as medians and quartiles. Results of the radiographic assessment are given as cross tabulations.
3 Results
3.1 Radiographs
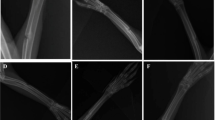
All doses of extracts with bioglass increased bone formation (BF) observed on radiographs compared with the bioglass alone and untreated groups (P < 0.002 to P < 0.03). With extract doses of 10–40 mg + bioglass, bone formation was superior as soon as 3 weeks postoperatively when compared with the plain bioglass and untreated groups (P < 0.03). Details of the BF data evaluated by radiographical analysis 3–8 weeks after the surgery are presented in Table 1. Typical radiographs showing new bone formation in different study groups after 8 weeks are shown in Fig. 1.
Radiographs showing new bone formation induced by different amounts of reindeer bone protein extract in the rat femur after 8 weeks follow-up. a: 5 mg of bone protein extract, b: 10 mg of bone protein extract, c: 15 mg of bone protein extract, d: 40 mg of bone protein extract, e: bioglass, f: untreated defect
Bone union (BU) at 6 weeks was better in the groups receiving bioglass + 10–40 mg of the extract compared with the plain bioglass and untreated groups (P < 0.009 to P < 0.03, respectively). Detailed BU data of are presented in Table 2.
3.2 pQCT
pQCT showed cross-sectional bone areas to be greater in the BG + 15 mg of the extract group and in the bioglass alone group than in the untreated group (P < 0.03) (Table 3). Bone density at the defect site was greater in all groups that received bone protein extract with bioglass and the bioglass alone group than in the untreated group (P < 0.004). There were no significant difference in bone density between the plain bioglass group and any of the extract groups (Table 3).
3.3 Torsion testing
Torsional stiffness of the bone was marginally higher in the group receiving BG + 15 mg of extract compared with the untreated group (P = 0.066) (Table 3; Figs. 2, 3).
4 Discussion
This study shows for the first time that bioglass is a candidate carrier for reindeer bone protein extract when used in a rat femur defect model. The results suggest that bone extract enhances the healing of the bone, compared with bioglass alone, 8 weeks after lesion formation, which is in agreement with previously published studies. Although bioglass is used alone in bone surgery, its healing effect is increased when it is combined with BMP products [15, 16, 18, 19, 31, 32] or with other inorganic materials like tricalcium phosphate (TCP) or a calcium sulphate barrier [13, 33], or with various organic materials [32, 34–37].
This was the first test of bioglass granules combined with reindeer bone protein extract. Previously, bioglass has been used as a carrier for bovine bone extract, demineralised bone matrix, and allografts [18, 19, 31]. Many other inorganic carrier materials have been used with native reindeer bone extracts. Pekkarinen et al. [24] used TCP, hydroxyapatite (HA), and coral as scaffolds for native reindeer bone extract in a mouse model of ectopic bone formation. TCP cylinders and coral composite implants with moose bone protein extract and type IV collagen have been used in the sheep segmental defect model [38, 39]. Canine segmental defects have been treated with bovine bone extract and HA or coral [40, 41]. Bovine bone extract and Plaster of Paris have been used clinically to treat femoral shaft non-unions in patients in a study by Bai et al. [42], and in scaffold and ulnar non-union with coral implants in a study by Kujala et al. [43, 44]. Potential advantages of bioglass over HA, TCP, or polymers include its superior biomechanical strength, higher osteoconduction, biocompatibility, and faster degradation [9, 45–47].
For about 20 years, our research group has studied the osteoinductivity of extracted reindeer bone protein (containing BMPs). We have shown that reindeer bone protein extract is an effective inducer of new bone formation in a muscle pouch model in mice [21, 22, 24, 25]. Furthermore, the good healing capacity of the reindeer bone extract with a collagen carrier in a segmental bone defect was previously demonstrated in the rabbit [26]. BMPs have been extracted from the bone materials of a variety of animal species, humans, and from bone tumours. The ability of reindeer bone protein extract to heal various bone traumas has been noted as being better than bovine or ostrich BMP extract, for example, which is attributed to reindeers’ ability to renew their antlers annually [20, 48]. Furthermore, it was suggested that more of the protein material extracted from the reindeer bone is in monocomponent form compared with that of bovine, ovine, or porcine protein material [20]. Surprisingly, the effect of native reindeer bone extract in the rat femur defect model was not as good as in the rabbit ulnar defect model owing to differences in animal species and experimental model [26]. It could also imply that native reindeer bone extract is immunogenic for the rat model [49, 50]. The used doses of native bone extracts are milligrams compared to recombinant products that are in micrograms. This is because the bone extracts and demineralised bone matrix products involve a wide spectrum of all bone proteins and collagens while recombinants only one specific protein with specific task in bone regeneration [51, 52]. The difference in the response of recombinant and native reindeer bone extract might partly be explained by the different effective doses, and more studies on the optimal dosing are needed.
An ideal bone implant would be osteoconductive, osteoinductive, radiolucent, and resorbable to allow easy radiographic evaluation of bone formation and healing [3, 5, 7, 53]. The bone graft substitute should also avoid being rejected or inducing a foreign body response [27] and should retain BMPs at the implant site for a period of time sufficient to induce bone formation [15]. Some properties of bioglass granules can affect the bone-healing capacity of BMP extracts. In this study, we used traditional bioglass granules. SiO2 (53% in bioglass granules used in the present study) worked as a network former, and had a slow and incomplete resorption property. Na2O (23%), CaO (20%), and P2O5 (4%) are compounds found in bioglass granules, and the variation in their ratios can be changed to adjust the dissolution rate [30, 31, 54, 55]. Variations in local pH levels also strongly influence the osteoclastic activity and reactions of bioglass in bone trauma [56].
Granulometry is influenced by the biological properties of bioactive glass [9, 13–15, 57, 58]. The size of the bioglass granules used in this study was 500–800 μm, which appeared to be a good size because the granules could be impacted firmly and stably in the bone defect and because granules of this size are not resorbed too quickly. In our preliminary study (results not shown) we used smaller granule size of bioglass (<300 μm), but after 3 weeks the granules had resorbed, and no bone formation could be seen even after 8 weeks. Different ratios of the compounds of bioglass granules could have been used to adjust the degradation process, allowing more time for osteoconduction and a larger pore size would also have yielded slower resorption of the bioglass; thus pore sizes > 300 μm are recommended [30, 58–60].
We used an 8-week follow-up time in this study. However, because bioglass resorbs slowly, we decided to extend the follow-up time of the bioglass + 40 mg extract group from 8 to 10 weeks to assess longer-term healing. The results of bone formation and bone union in this group were significantly better after 10 weeks compared with all the other groups after 8 weeks follow-up. However, there was plenty of bioglass material remaining even after 10 weeks follow-up when granule sizes of 500–800 μm were used. It is known that bioglass is radiopaque and can have influence on the pQCT results, the density of bioglass being slightly higher than that of bone [31]. This may explain why there were no statistically significant differences in bone density between any implant groups, although the bone extract groups displayed more bone formation than bioglass group (which displayed the highest density value). Thus, bioglass may overcome the density of new bone. On the other hand, plain radiographs showed signs of increased resorption of bioglass in the bone extract groups which could decrease the density values.
Furthermore, the lack of histological quantification could have limited the estimation of the results and comparing the bone extract groups to control groups. A longer follow-up time should be used when bioglass is used as the carrier compared with collagen, for example [15]. We also found that in the group 40 mg + BG biomechanical results were better after 10 weeks follow-up than in each other groups (these results not shown).
In this study, 10 rats died soon after surgery of no obvious cause. No relationship with bone extract or bioglass could be inferred, because deaths also occurred in the control groups. The deaths may have been reactions to anaesthesia, although the anaesthesia and methods used are well accepted in animal testing. Upon necropsy, some rats that died showed signs of pulmonary oedema that is a typical phenomenon after great bone surgery.
Native bone protein extract proved to be functional in the bioglass carrier. Theoretically, particles containing bone proteins could adhere to the surface of the bioglass granules and be released when the bioglass granules dissolve around the defect. The bone-healing effect of native bone extract combined with bioglass granules was encouraging, but the optimization of the carrier and formulated bone protein extract is required before native reindeer bone protein extract implants are ready for clinical use.
5 Conclusions
Bioglass granules seem to perform well as a carrier of reindeer bone protein extract at doses of bioglass + 10–40 mg, and a dose-dependent effect of the extract on the healing of the bone was observed.
References
Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003;85-A(8):1544–52. Erratum in: J Bone Joint Surg Am. 2004;86-A(1):144.
Xiao YT, Xiang LX, Shao JZ. Bone morphogenetic protein. Biochem Biophys Res Commun. 2007;362(3):550–3.
Kirker-Head CA. Potential applications and delivery strategies for bone morphogenetic proteins. Adv Drug Deliv Rev. 2000;43(1):65–92.
Li RH, Wozney JM. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001;19(7):255–65.
Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm. 2004;58(2):197–208.
Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopaedic tissue regeneration. Cytokine Growth Factor Rev. 2005;16(3):329–45.
Saito N, Takaoka K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials. 2003;24:2287–93.
Jones JR, Ehrenfried LM, Hench LL. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials. 2006;27:964–73.
Oonishi H, Hench LL, Wilson J, Sugihara F, Tsuji E, Kushitani S, et al. Comparative bone growth behaviour in granules of bioceramic materials of various sizes. J Biomed Mater Res. 1999;44:31–43.
Silver IA, Deas J, Erecińska M. Interactions of bioactive glasses with osteoblasts in vitro: effects of 45S5 Bioglass®, and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomaterials. 2001;22:175–85.
Pyhältö T, Lapinsuo M, Pätiälä H, Rokkanen P, Niiranen H, Törmälä P. Fixation of distal femoral osteotomies with self-reinforced poly(l/dl)lactide 70:30/bioactive glass composite rods. An experimental study on rats. J Mater Sci: Mater Med. 2004;15:275–81.
Hench LL. The story of Bioglass®. J Mater Sci: Mater Med. 2006. doi:10.1007/s10856-006-0432-z.
Ghosh SK, Nandi SK, Kundu B, Datta S, De DK, Roy SK, et al. In vivo response of porous hydroxyapatite and β-tricalcium phosphate prepared by aqueous solution combustion method and comparison with bioglass scaffolds. J Biomed Mater Res B: Appl Biomater. 2008;86B:217–27.
Mahmood J, Takita H, Ojima Y, Kobayashi M, Kohgo T, Kuboki Y. Geometric effect of matrix upon cell differentiation: BMP-induced osteogenesis using a new bioglass with a feasible structure. J Biochem. 2001;129(1):163–71.
Takita H, Vehof JWM, Jansen JA, Yamamoto M, Tabata Y, Tamura M, et al. Carrier dependent cell differentiation of bone morphogenetic protein-2 induced osteogenesis and chondrogenesis during the early implantation stage in rats. J Biomed Mater Res. 2004;71A:181–9.
Välimäki VV, Yrjans JJ, Vuorio E, Aro HT. Combined effect of BMP-2 gene transfer and bioactive glass microspheres on enhancement of new bone formation. J Biomed Mater Res. 2005;75A:501–9.
Bergeron E, Marquis ME, Chrétien I, Faucheux N. Differentiation of preosteoblasts using a delivery system with BMPs and bioactive glass microspheres. J Mater Sci: Mater Med. 2007;18(2):255–63.
Pajamäki KJ, Andersson OH, Lindholm TS, Karlsson KH, Yli-Urpo A, Happonen RP. Effect of bovine bone morphogenetic protein and bioactive glass on demineralised bone matrix grafts in the rat muscular pouch. Ann Chir Gynaecol Suppl. 1993;207:155–61.
Pajamäki KJ, Andersson OH, Lindholm TS, Karlsson KH, Yli-Urpo A. Induction of new bone allogeneic demineralised bone matrix combined to bioactive glass composite in the rat. Ann Chir Gynaecol Suppl. 1993;207:137–43.
Jortikka L, Marttinen A, Lindholm TS. Partially purified reindeer (Rangifer Tarandus) bone morphogenetic protein has a high bone-forming activity compared with some other artiodactylis. Clin Orthop Relat Res. 1993;297:33–7.
Pekkarinen T, Lindholm TS, Hietala O, Jalovaara P. New bone formation induced by injection of native reindeer BMP. Scand J Surg. 2003;92:227–30.
Pekkarinen T, Lindholm TS, Hietala O, Jalovaara P. Influence of ethylene oxide sterilization on the activity of native reindeer bone morphogenetic protein. Int Orthop. 2004;28(2):97–101.
Pekkarinen T, Lindholm TS, Hietala O, Jalovaara P. The effect of different mineral frames on the ectopic bone formation in mouse hind leg muscles induced by native reindeer bone morphogenetic protein. Arch Orthop Trauma Surg. 2005;125(1):10–5.
Pekkarinen T, Hietala O, Jämsä T, Jalovaara P. Effect of gamma irradiation on the osteoinductivity of native reindeer bone morphogenetic protein extract. Acta Orthop. 2005;76(2):231–6.
Pekkarinen T, Hietala O, Jämsä T, Jalovaara P. Gamma irradiation and ethylene oxide in the sterilization of native reindeer bone morphogenetic protein extract. Scand J Surg. 2005;94(1):67–70.
Pekkarinen T, Jämsä T, Määttä M, Hietala O, Jalovaara P. Reindeer BMP extract in the healing of critical-size bone defects in the radius of the rabbit. Acta Orthop. 2006;77(6):952–9.
Sciadini MF, Dawson JM, Johnson KD. Bovine-derived bone protein as a bone graft substitute in a canine segmental defect model. J Orthop Trauma. 1997;11(7):496–508.
Lepola V, Väänänen K, Jalovaara P. The effect of immobilization on the torsional strength of the rat tibia. Clin Orthop Relat Res. 1993;297:55–61.
Jämsä T, Jalovaara P. A cost-effective, accurate machine for testing the torsional strength of sheep long bones. Med Eng Phys. 1996;18(5):433–5.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300.
Griffon DJ, Dunlop DG, Howie CR, Gilchrist T, Salter DM, Healy DM. Early dissolution of a morsellised impacted silicate-free bioactive glass in metaphyseal defects. J Biomed Mater Res (Appl Biomater). 2001;58:638–44.
Livingston T, Ducheyne P, Garino J. In vivo evaluation of a bioactive scaffold for bone tissue engineering. J Biomed Mater Res. 2002;62:1–13.
Melo LGN, Nagata MJH, Bosco LLG, Leite CM. Bone healing in surgically created defects treated with either bioactive glass particles, a calcium sulphate barrier, or a combination of both materials. A histological and histometric study in rat tibias. Clin Oral Implants Res. 2005;16:683–91.
Kotani S, Yamamuro T, Nakamura T, Kitsugi T, Fujita Y, Kawanabe K, et al. Enhancement of bone bonding to bioactive ceramics by demineralized bone powder. Clin Orthop Relat Res. 1992;278:226–34.
Shinzato S, Kobayashi M, Mousa WF, Kamimura M, Neo M, Kitamura Y, et al. Bioactive polymethyl methacrylate-based bone cement: comparison of glass beads, apatite- and wollastonite-containing glass-ceramic, and hydroxyapatite fillers on mechanical and biological properties. J Biomed Mater Res. 2000;51:258–72.
Meretoja VV, Helminen AO, Korventausta JJ, Haapa-aho V, Seppälä JV, Närhi TO. Crosslinked poly(ε-caprolactone/d, l-lactide)/bioactive glass composite scaffolds for bone tissue engineering. J Biomed Mater Res. 2006;77A:261–8.
Chen QZ, Ahmed I, Knowles JC, Nazhat SN, Boccaccini AR, Rezwan K. Collagen release kinetics of surface functionalized 45S5 Bioglass®-based porous scaffolds. J Biomed Mater Res. 2008;86A:987–95.
Gao TJ, Lindholm TS, Kommonen B, Ragni P, Paronzini A, Lindholm TC, et al. Enhanced healing of segmental tibial defects in sheep by a composite bone substitute composed of tricalcium phosphate cylinder, bone morphogenetic protein, and type IV collagen. J Biomed Mater Res. 1996;32(4):505–12.
Gao TJ, Lindholm TS, Kommonen B, Ragni P, Paronzini A, Lindholm TC, et al. The use of a coral composite implant containing bone morphogenetic protein to repair a segmental tibial defect in sheep. Int Orthop. 1997;21(3):194–200.
Tuominen T, Jämsä T, Tuukkanen J, Nieminen P, Lindholm TC, Lindholm TS, et al. Native bovine bone morphogenetic protein improves the potential of biocoral to heal segmental canine ulnar defects. Int Orthop. 2000;24(5):289–94.
Tuominen T, Jämsä T, Oksanen J, Tuukkanen J, Gao TJ, Lindholm TS, et al. Composite implant composed of hydroxyapatite and bone morphogenetic protein in the healing of a canine ulnar defect. Ann Chir Gynaecol Suppl. 2001;90(1):32–6.
Bai MH, Liu XY, Ge BF, Yallag C, Chen DA. An implant of a composite of bovine bone morphogenetic protein and plaster of paris for treatment of femoral shaft nonunions. Int Surg. 1996;81(4):390–2.
Kujala S, Raatikainen T, Ryhänen J, Kaarela O, Jalovaara P. Composite implant of native bovine bone morphogenetic protein (BMP) and biocoral in the treatment of scaphoid nonunions—a preliminary study. Scand J Surg. 2002;91(2):186–90.
Kujala S, Raatikainen T, Ryhänen J, Kaarela O, Jalovaara P. Composite implant of native bovine bone morphogenetic protein (BMP), collagen carrier and biocoral in the treatment of resistant ulnar nonunions: report of five preliminary cases. Arch Orthop Trauma Surg. 2004;124:26–30.
Kawanabe K, Tamura J, Yamamuro T, Nakamura T, Kokubo T, Yoshihara S. A new bioactive bone cement consisting of BIS-GMA resin and bioactive glass powder. J Appl Biomater. 1993;4(2):135–41.
Marcolongo M, Ducheyne P, Garino J, Schepers E. Bioactive glass fiber/polymeric composites bond to bone tissue. J Biomed Mater Res. 1998;39:161–70.
Chen QZ, Thompson ID, Boccaccini AR. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials. 2006;27:2414–25.
Ulmanen MS, Pekkarinen T, Hietala OA, Birr EA, Jalovaara P. Osteoinductivity of partially purified native ostrich (Struthio camelus) bone morphogenetic protein: comparison with mammalian species. Life Sci. 2005;77(19):2425–37.
Granjeiro JM, Oliveira RC, Bustos-Valenzuela JC, Sogayar MC, Taga R. Bone morphogenetic proteins: from structure to clinical use. Braz J Med Biol Res. 2005;38:1463–73.
Sampath TK, Reddi AH. Homology of bone-inductive proteins from human, monkey, bovine and rat extracellular matrix. Proc Natl Acad Sci USA. 1983;80(21):6591–5.
Baas J, Lamberg A, Jensen TB, Elmengaard B, Søballe K. The bovine bone protein lyophilisate colloss improves fixation of allografted implants—an experimental study in dogs. Acta Orthop. 2006;77(5):791–8.
Nienhuijs MEL, Walboomers XF, Merkx MAW, Stoelinga PJW, Jansen JA. Bone-like tissue formation using an equine COLLOS® E-filled titanium scaffolding material. Biomaterials. 2006;27:3109–14.
Winn SR, Uludag H, Hollinger JO. Carrier systems for bone morphogenetic proteins. Clin Orthop Relat Res. 1999;367S:S95–106.
Zhong J, Greespan DC. Processing and properties of sol-gel bioactive glasses. J Biomed Mater Res (Appl Biomater). 2000;53:694–701.
Moura J, Teixeira LN, Ravagnani C, Peitl O, Zanotto ED, Beloti MM, et al. In vitro osteogenesis on a highly bioactive glass-ceramic (Biosilicate®). J Biomed Mater Res. 2007;82A:545–57.
Cerruti M, Greespan D, Powers K. Effect of pH and ionic strength on the reactivity of Bioglass® 45S5. Biomaterials. 2005;26:1665–74.
Schepers EJG, Ducheyne P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: a 1–24 month experiment with several materials and particle sizes and size ranges. J Oral Rehabil. 1997;24(3):171–81.
Wheeler DL, Stokes KE, Hoellrich RG, Chamberland DL, McLoughlin SW. Effect of bioactive glass particle size on osseous regeneration of cancellous defects. J Biomed Mater Res. 1998;41:527–33.
Hall EE, Meffert RM, Hermann JS, Mellonig JT, Cochran DL. Comparison of bioactive glass to demineralised freeze-dried bone allograft in the treatment of intrabony defects around implants in the canine mandible. J Periodontol. 1999;70(5):526–35.
Karageorgiour V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91.
Acknowledgment
The authors would like to thank to the Synthes Company and Mr. Antero Enqvist for providing Kirschner wires; the Vivoxid Company and PhD Ilkka Kangasniemi for providing bioglass for this study; and Bioactive Bone Substitutes (BBS) Ltd for providing native reindeer bone protein extracts. We are grateful to PhD Oili Hietala, and Mrs. Merja Haikola for their help with the carrier planning, Ms. Heli Korkala for her assistance with surgery, and Mr. Risto Bloigu for statistical assistance. The study was partly supported by Academy of Finland and National Graduate School of Musculoskeletal Disorders and Biomaterials (TBGS, Finland).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tölli, H., Kujala, S., Levonen, K. et al. Bioglass as a carrier for reindeer bone protein extract in the healing of rat femur defect. J Mater Sci: Mater Med 21, 1677–1684 (2010). https://doi.org/10.1007/s10856-010-4017-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4017-5