Abstract
Introduction
Bone morphogenetic proteins (BMPs) require carrier material for slow release and framing material for osteoconduction.
Materials and methods
The effect of a frame on early bone formation induced by partially purified native reindeer BMP in composite implants containing 3 mg of BMP, type IV collagen and tricalcium phosphate (TCP/Col/BMP) or hydroxyapatite (HA/Col/BMP) or biphasic tricalcium phosphate-hydroxyapatite (TCP/HA/Col/BMP) or biocoral (NC/Col/BMP) was evaluated using a mouse hind leg muscle pouch model. Collagen with native reindeer BMP (Col/BMP) and corresponding implants without native reindeer BMP served as controls. Evaluation was done by incorporation of 45Ca, radiographically and histologically 3 weeks after the implantation.
Results
None of the implants without native reindeer BMP were able to induce new bone visible on radiographs. The area of new bone formation in the Col/BMP (p=0.026) and TCP/HA/Col/BMP (p=0.012) groups was significantly greater than in the TCP/Col/BMP group. The optical density of the new bone area was significantly greater in the TCP/HA/Col/BMP group than in the TCP/Col/BMP (p=0.036) or Col/BMP (p=0.02) groups. 45Ca incorporation was many times greater in all the groups containing native reindeer BMP than in the corresponding groups without BMP. In the Col/BMP (p=0.046) and TCP/HA/Col/BMP (p=0.046) groups, 45Ca incorporation was significantly greater than in the TCP/Col/BMP group. No significant differences were found in any parameters between HA/Col/BMP and NC/Col/BMP groups and the other BMP-containing groups.
Conclusions
Hydroxyapatite, biocoral and biphasic tricalciumphosphate-hydroxyapatite are equally good as framing material for native reindeer BMP, while tricalciumphosphate is somewhat worse. Osteoinduction of native reindeer BMP works well with collagen alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone morphogenetic proteins (BMPs) constitute a large family of proteins which are osteoinductive and able to produce bone at ectopic sites [4, 29]. BMPs have been used to heal bone defects in experimental animal tests and clinical settings, mainly in orthopaedic and oral surgery [10, 11, 15, 25, 31, 32]. BMPs need a delivery system to be successfully active in the target tissue. A variety of inorganic materials, including coralline, hydroxyapatite, tricalcium phosphate, organic materials such as collagens, and allografts, have been successfully used as delivery materials for BMPs in animal experiments [1, 2, 5, 6, 8, 9, 16, 17, 20, 21, 26, 27, 28, 30]. However, knowledge of the suitability of these materials is insufficient, and studies comparing several frames simultaneously are needed.
In this study, we evaluated the effects of tricalcium phosphate, hydroxyapatite, biphasic tricalcium phosphate-hydroxyapatite and natural coral frames on the early osteoinduction of native reindeer BMP in a femoral muscle pouch of a mouse model.
Materials and methods
BMP was prepared from reindeer diaphyseal bones. Cortical bones from each animal were chilled immediately after death. The epiphyseal ends, bone marrow and periosteum were mechanically removed, and the cleaned cortical bones were sawn and, after freezing in liquid nitrogen, ground to a particle size of 1.0 mm3. The bone matrix was extracted in 4 M Guanidine hydrochloride (GuHCl) at 4oC after the pulverized bone had been demineralized in 0.6 mol/l HCl. The GuHCl-extracted solution was filtered by a tangential flow system and concentrated. The concentrated solution was dialysed against deionized water, and the water-insoluble material was collected. After being redissolved in 4 M GuHCl solution, the water-insoluble material was dialyzed against 0.25 M citrate buffer, pH 3.1. The citrate-buffer-insoluble material was washed with deionized water and lyophilized [12].
The collagen sponge (Col; weight 15 mg, Lyostypt®, compress from native collagen of bovine origin, mainly consisting of type IV collagen; B. Braun, Tuttlingen, Germany) was used as a carrier of native reindeer BMP.
Four different framing materials were tested as part of the composite implant:
-
1.
Tricalcium phosphate [Ca3(PO4)2], TCP (Bioland, Toulouse, France). The size of the frame was 3×3x4 mm and average weight 30 mg. The diameters of the pores were 200–500 μm with 70% porosity.
-
2.
Hydroxyapatite (Ca10[PO4]6[OH]2), HA (Bioland, Toulouse, France). The size of the frame was 3×3x4 mm and average weight 30 mg. The diameters of the pores were 200–500 μm with 70% porosity.
-
3.
Biphasic tricalcium phosphate and hydroxyapatite frame, TCP/HA (Bioland, Toulouse, France). The size of the frame was 3×3x4 and average weight 30 mg. The frames consisted of 25% hydroxypatite and 75% tricalcium phosphate. The diameters of the pores were 200–500 μm with 70% porosity.
-
4.
Natural coral, NC (Biocoral®, Inoteb, St Connery, France). The frames were symmetrical balls 3 mm in diameter, and their average weight was 40 mg. Coral, the limestone skeleton of various species of marine invertebrates, consists mainly of calcium carbonate (>98%, CaCO3). The rest includes such elements as fluoride, strontium, magnesium and amino acids. The mean diameter of the macropores was 150 μm with 50% porosity.
According to our preliminary test, 3 mg of reindeer BMP induce a suitable amount of new bone and was therefore used in all composite implants (Fig. 1). The composite implants were constructed by pipetting 3 mg of native reindeer BMP extract onto the collagen sponge. After that, the frames were embedded with collagen sponge that either did or did not contain BMP. Finally, the implants were lyophilized in test tubes for 48 h. A carrier material (collagen) without frames was also tested.
Male NMRI mice aged 28–35 weeks were used. The implants were introduced under sterile conditions into the thigh muscle pouches of the bilateral hind legs under neuroleptic analgesia (Hypnorm®, Janssen, Belgium; Dormicum®, Roche, Swiss). After implantation, the muscle was closed with 5–0 and the skin with 3–0 resorbable sutures. The animals were killed in a chamber after 21 days of implantation using carbon dioxide, and the hind legs were harvested [12, 24]. The study protocol was approved by the Ethical Committee of the University of Oulu.
The study and control groups were named according to the composite implants used.
Study groups:
-
1.
Tricalcium phosphate + collagen + 3 mg of BMP (TCP/Col/BMP) (10 implants)
-
2.
Hydroxyapatite + collagen + 3 mg of BMP (HA/Col/BMP) (10 implants)
-
3.
Tricalcium phosphate + hydroxyapatite + collagen + 3 mg of BMP (TCP/HA/Col/BMP) (10 implants)
-
4.
Natural coral + collagen + 3 mg of BMP (NC/Col/BMP) (10 implants)
Control groups:
-
5.
Tricalcium phosphate + collagen (TCP/Col) (6 implants)
-
6.
Hydroxyapatite + collagen (HA/Col) (6 implants)
-
7.
Tricalcium phosphate + hydroxyapatite + collagen (TCP/HA/Col) (6 implants)
-
8.
Natural coral + collagen (NC/Col) (6 implants)
-
9.
Collagen (Col) (6 implants)
-
10.
Collagen + 3 mg of BMP (Col/BMP) (10 implants)
New bone formation and its density evaluated by radiographs
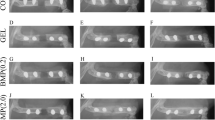
After the harvest, standard lateral position radiographs (100 mA, 20 kV, 0.08 s/exp; Mamex de Maq, Soredex, Orion) were taken of all mouse hind legs. The radiographic images were transferred into a computer by using an optical scanner (HP Laser Jet/Desk Scan). New bone formation was evaluated as the area (mm2) of calcified tissue visible in the radiographs defined by using Scion Image software (Scion, USA). In the cases where the implant contained radiopaque frames, the pheripheral calcified tissue around the frame was measured (Fig. 2). The mean optical density (mmAl) of the defined area was measured with the same equipment. The calibration of equipment for the measurement of optical density was performed using an aluminium wedge.
Measurement of 45Ca activity
Twenty-four hours before the animals were killed, four randomly selected mice in each test group (eight implants) and two mice in each control group (four implants) received an intraperitoneal injection of diluted carrier-free 45Ca solution (Amersham, UK; 40 µCi/kg of body weight). The muscle tissue of each harvested hind leg including an implant and newly formed bone was taken en bloc for a specimen immediately after the radiography. A piece of intact foreleg muscle was used as reference (5 samples). All specimens were weighed and digested in a mixture of 0.2 ml 70% perchloric acid and 0.4 ml 33% peroxide at 70oC for 3 h. Then 0.6 ml of digested solution was pipetted into a diffuse scintillation vial, and 5 ml scintillation cocktail (OptiPhase’Hi-safe 3‘,Wallac, Finland) was added. The samples were counted in a liquid scintillation counter (Wallac 1410, Pharmacia, Finland) with an internal spectrum library. 45Ca incorporation into tissue was expressed as DPM/mg of tissue.
Histological examination
The hind legs of one mouse (two implants) in each group were used for histological examination. The specimens were prepared as described above, fixed in 10% neutral formalin solution, decalcified in 0.1 N HCl, cut at 2.0-mm intervals into 5-μm-thick sections and stained with haematoxylin & eosin.
Statistical analysis
The non-parametric Kruskal-Wallis test was used to evaluate the statistical differences between the groups and the Mann-Whitney test for pairwise comparisons. Statistical analysis was performed using the SPSS for Windows statistical package (SPSS, version 9.0). Values of p<0.05 were considered statistically significant.
Results
Area of new bone formation evaluated by radiography
Implants without native reindeer BMP were not able to induce new bone visible in radiographs (Table 1). Also the Mann-Whitney U-test showed new bone formation to be significantly more abundant in the Col/BMP (p=0.026) and TCP/HA/Col/BMP (p=0.012) groups than in the TCP/Col/BMP group. No significant difference was found between the HA/Col/BMP and NC/Col/BMP groups and the other BMP-containing groups. New bone formation was most marked in the Col/BMP group and least abundant in the TCP/Col/BMP group (Table 1).
Density of new bone formation evaluated by radiography
Because the groups without native reindeer BMP showed no new bone formation, the densities could not be measured in these groups. The Mann-Whitney U-test showed that the density of new bone formation in the TCP/HA/Col/BMP group was significantly greater than that in the TCP/Col/BMP (p=0.036) and Col/BMP (p=0.02) groups. No significant difference was found between HA/Col/BMP and NC/Col/BMP groups and the other BMP-containing groups (Table 2). The density of new bone formation was most marked in the TCP/HA/Col/BMP group and least marked in the TCP/Col/BMP group.
45Ca incorporation
45Ca incorporation was manifold in all groups containing native reindeer BMP compared with the corresponding groups without native reindeer BMP (Kruskall-Wallis test, p=0.00) (Tables 3 and 4). 45Ca incorporation was significantly greater in the Col/BMP (p=0.046) and TCP/HA/Col/BMP (p=0.046) groups than in the TCP/Col/BMP group. No significant difference was found between HA/Col/BMP and NC/Col/BMP groups and the other BMP-containing groups (Table 3).
Histological analysis
Histological examination showed endochondral new bone formation in the specimens of all groups with native reindeer BMP but not in those without. The most abundant bone formation was seen in the specimens of Col/BMP, which showed substantial amounts of hypertrophied and calcified chondrocytes, remodelling ectopic trabecular woven bone and haematopoietic bone marrow cells. Some residuals of fibrotic collagen pellets could also be seen.
In the other groups with native reindeer BMP extract, the histological features were quite similar. The newly formed woven bone mainly occurred in small patches around the frames. The calcified cartilage and the connective tissue were dispersed around and deposited into the pores of the frames. The specimens of the TCP/Col/BMP group seemed to contain the most abundant amounts of fibrotic connective tissue and smaller amounts of cartilage and endochondral woven bone than the other groups. No inflammatory cells were seen.
Discussion
An ideal BMP delivery substrate should meet the following criteria: relative insolubility under physiological conditions, biodegradability, protection against proteolytic digestion, can act as a scaffold for cell adhesion and proliferation, immunological inertia, slow release of BMPs through controlled biological degradation [17]. The substrates should also be osteoconductive, practical and easy to use, and they should also have adequate mechanical strength for loading. Inorganic frame materials, such as coralline, hydroxyapatite, tricalcium phosphate and hydroxyapatite-tricalcium phosphate, have most of these properties and have been applied as frames for BMPs [1, 2, 5, 6, 8, 9, 16, 17, 20, 21, 26, 27, 28, 30].
The pore size of the frame has been shown to be associated with the quantity of newly formed bone through the growth of osteoblasts and the attachment and ingrowth of vessels [13, 14, 27]. Kawamura et al. [13] and Klawitter and Hulbert [14] have shown that a pore size of 100–200 μm is sufficient for osteoinduction, and Tsuruga et al. [27] reported the optimal pore size to be 300–400 μm. The pore sizes of 150 μm for biocoral and 200–500 μm for the other frames used in the present study are well in line with these previous reports and make our results comparable.
However, the frames do not work properly with BMPs alone, primarily because the release of BMPs is too rapid. A carrier that binds and releases BMPs slowly is required. Collagen is commonly used. When collagen is present, the BMPs concentration at the interface between the implant and the host tissue remains high, enabling the responding cells to come into contact with BMP molecules for long enough to initiate bone induction [19]. In addition to the delivery function, collagens have been shown to have favourable effects on the bone-forming activity of BMPs [3, 7, 18, 33]. Collagen acts as a temporary scaffold for bone formation and sets up chemotaxis for the aggregation of osteoprogenitor cells [17]. It has been observed that natural or denatured type IV collagen has a more potent affinity to BMP-2B and BMP-3 than the type I, II, IX and XI collagens [22, 23]. Because of this, carrier consisting of type IV collagen was used in this study.
Only the implants with native reindeer BMP showed induction of bone formation. This indicates that the native reindeer BMP used in this study was effective, and the carrier worked appropriately. The frames used did not have osteoinductive properties and proved to function solely as osteoconductive materials. However, the frames may have different effects on the osteoinductive capacity of BMPs.
Tricalcium phosphate has been used with BMPs with satisfactory outcomes [8, 16, 30]. Laffrague et al. [16] showed, by using a femoral condyle bone defect model in rabbits, that tricalcium phosphate is a serviceable matrix for recombinant BMP-2 and gives it osteoinductive properties in a dose-dependent manner. In our study, the tricalcium phosphate frame worked adequately but not as well as the other frames. This is in line with the study by Gao et al. [8], who used a study design similar to ours to show that natural coral worked better than tricalcium phosphate.
It should be noted that native reindeer BMP here induced equally good or better new bone formation with collagen alone than with any of the frames tested. BMPs have been successfully used with collagen to treat different experimental bone defects in animal models [18, 33]. It has been shown that composite implants with frames have more favourable effects on the osteoinductive capacity of BMPs than collagen alone [8], but this finding could not be confirmed in our study. However, the frames may be indicated for fixation, osteoconduction and mechanical loading. The composites containing hydroxyapatite, biphasic tricalciumphosphate-hydroxyapatite and biocoral frames seemed here to be roughly similar. Also, these frames have been used successfully in many previous studies with BMPs [1, 6, 8, 17, 21], but no other studies comparing several frames with each other could be found.
In conclusion, only the implants with native reindeer BMP were able to induce new bone formation in a mouse muscle pouch within 3 weeks. The effects of hydroxyapatite, biocoral and biphasic tricalciumphosphate-hydroxyapatite frames on the early osteoinduction of native reindeer BMP were of the same order, but the tricalcium phosphate frame seemed to be somewhat worse. On the other hand, collagen carrier alone with native reindeer BMP induced new bone equally well as the composites with the best frames.
References
Alam MI, Asahina I, Ohmamiuda K, Takahashi K, Yokota S, Enomoto S (2001) Evaluation of ceramics composed of different hydroxyapatite to tricalcium phosphate ratios as carriers for rhBMP-2. Biomaterials 22:1643–1651
Arnaud E, Pollack C, Meunier A, Sedel L, Damien C, Petite H (1999) Osteogenesis with coral is increased by BMP and BMC in a rat cranioplasty. Biomaterials 20:1909–1918
David SM, Gruber HE, Meyer RA Jr, Murakami T, Tabor OB, Howard BA, Wozney JM, Hanley EN Jr (1999) Lumbar spinal fusion using recombinant human bone morphogenetic protein in the canine. A comparison of three dosages and two carriers. Spine 24:1973–1979
Ebara S, Nakayama K (2002) Mechanism for the action of bone morphogenetic proteins and regulation of their activity. Spine 27:10–15
Gao TJ, Tuominen TK, Lindholm S, Kommonen B, Lindholm TC (1997) Morphological and biomechanical differences in healing in segmental tibial defects implanted with biocoral or tricalcium phosphate cylinders. Biomaterials 18:219–223
Gao TJ, Lindholm TS, Kommonen B, Ragni P, Paronzini A, Lindholm TC, Jalovaara P, Urist MR (1997) A coral composite implant containing bone morphogenetic protein repairs a segmental tibial defect in sheep: mechanics and immune assay. Int Orthop 21:194–200
Gao TJ, Lindholm TS, Marttinen A, Puolakka T (1993) Bone inductive potential and dose-dependent response of bovine bone morphogenetic protein combined with type IV collagen carrier. Ann Chir Gyn 82:77–84
Gao TJ, Lindholm TS, Marttinen A, Urist MR (1996) Composite of bone morphogenetic protein (BMP) and type IV collagen, coral-derived coral hydroxyapatite and tricalcium phosphate ceramics. Int Orthop 20:321–325
Jin QM, Takita H, Kohgo T, Atsumi K, Itoh H, Kuboki Y (2000) Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J Biomed Mater Res 51:491–499
Johnson EE, Urist MR (2000) Human bone morphogenetic protein allografting for reconstruction of femoral non-union. Clin Orthop 371:61–74
Johnson EE, Urist MR (1998) One stage lengthening of femoral nonunion augmented with human bone morphogenetic protein. Clin Orthop 347:105–116
Jortikka L, Marttinen A, Lindholm TS (1993) Purification of monocomponent bovine morphogenetic protein in a water-soluble form. Ann Chir Gyn 82:25–30
Kawamura M, Iwata H, Sato K, Miura T (1987) Chondroosteogenetic response to crude bone matrix proteins bound to hydroxyapatite. Clin Orthop 217:281–292
Klawitter JJ, Hulbert SF (1971) Application of porous ceramics for attachment of load bearing internal orthopedic applications. J Biomed Mater Res 2:161–229
Kujala S, Raatikainen T, Ryhänen J, Kaarela O, Jalovaara P (2002) Composite implant of native bovine bone morphogenetic protein (BMP) and biocoral in the treatment of scaphoid nonunions—a preliminary study. Scand J Surg 9:186–190
Laffrague P, Hildebrand HF, Rtaimate M, Frayssinet P, Amoureux JP, Marchandise (1999) Evaluation of human recombinant bone morphogenetic protein-2-loaded tricalcium phosphate implants in rabbits’ bone defect. Bone 25:55–58
Lindholm TS, Gao TJ (1993) Functional carriers for bone morphogenetic proteins. Ann Chir Gyn 82:3–12
Murata M, Huang BZ, Shibata T, Imai S, Nagai N, Arisue M (1999) Bone augmentation by recombinant human BMP-2 and collagen on adult rat parietal bone. Int Oral Maxillofac Surg 28:232–237
Nakahara H, Takaoka K, Koezuka M, Sugamoto K, Tsuda T, Ono K (1989) Periosteal bone formation elicited by partially purified BMP. Clin Orthop 239:299–305
Noshi T, Yoshikawa T, Ikeuchi M, Dohi Y, Ohgushi H, Horiuchi K, Sugimura M, Ichijima K, Yonemasu K (2000) Enhancement of the in vivo osteogenic potential of marrow/hydroxyapatite composites by bovine bone morphogenetic protein. J Biomed Mater Res 52:621–630
Okubo Y, Bessho K, Fujimura K, Kusumoto K, Ogawa Y, Izuka T (2000) Osteogenesis by recombinant human bone morphogenetic protein-2 at skeletal sites. Clin Orthop 375:295–301
Paralkar VM, Weeks BS, Yu YM, Leinman HK, Reddi AH (1992) Recombinant human bone morphogenetic protein 2B stimulates PC 12 cell differentiation: potentiation and binding to type IV collagen. J Cell Biol 119:1721–1728
Reddi AH (1992) Regulation of cartilage and bone differentiation by bone morphogenetic protein. Curr Opin Cell Biol 4:850–855
Reddi AH (1981) Cell biology and biochemistry of endochondral bone development. Coll Relat Res 1:209–226
Sailer HF, Kolb E (1994) Application of purified bone morphogenetic protein (BMP) in cranio-maxillo-facial surgery. BMP in compromised surgical reconstructions using titanium implants. J CranioMaxillofac Surg 22:2–11
Sheerman H, Wozney J, Li R (2002) Bone morphogenetic protein delivery systems. Spine 27:16–23
Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y (1997) Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem 121:317–324
Tuominen T, Jämsä T, Tuukkanen J, Nieminen P, Lindholm TC, Lindholm TS, Jalovaara P (2000) Native bone morphogenetic protein improves the potential of biocoral to heal bone segmental canine ulnar defects. Int Orthop 24:289–294
Urist MR (1965) Bone formation by autoinduction. Science 150:893–899
Urist MR, Lietze A, Dawson E (1984) Beta-tricalcium phosphate delivery system for bone morphogenetic protein. Clin Orthop 187:277–280
Valentin-Opran A, Wozney J, Csimma C, Lilly L, Riedel G (2002) Clinical evaluation of recombinant human bone morphogenetic protein-2. Clin Orthop 395:110–120
Wozney J (2002) Overview of bone morphogenetic proteins. Spine 27(16S):2–8
Yudell RM, Block MS (2000) Bone gap healing in the dog using recombinant human bone morphogenetic-2. J Oral Maxillofac Surg 58:761–766
Acknowledgements
We would like to profusely thank Mrs Anna-Liisa Siponen and Mr Aulis Marttinen in the Laboratory of the Department of Clinical Medicine, University of Tampere, for their kind assistance with the nucleic techniques and Bioactive Bone Substitutes Ltd, Oulu, Finland. The experiment complies with the current laws of Finland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pekkarinen, T., Lindholm, T.S., Hietala, O. et al. The effect of different mineral frames on ectopic bone formation in mouse hind leg muscles induced by native reindeer bone morphogenetic protein. Arch Orthop Trauma Surg 125, 10–15 (2005). https://doi.org/10.1007/s00402-004-0761-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-004-0761-7