Abstract
Cartilage extracellular matrix (ECM) is composed primarily of type II collagen (COL II) and large, networks of proteoglycans (PGs) that contain glycosaminoglycans such as hyaluronic acid (HA) and chondroitin sulfate (CS). Since cartilage shows little tendency for self-repair, injuries are kept unhealed for years and can eventually lead to further degeneration. During the past decades, many investigations have pursued techniques to stimulate articular cartilage repair or regeneration. The current study assessed the effects of exogenous glycosaminoglycans (GAGs) including CS-A, CS-B, CS-C, heparan sulfate and HA, administration on human chondrocytes in terms of proliferation and matrix synthesis, while the cells were seeded and grown on the genipin-crosslinked collagen type II (COL II) scaffold. DNA content was measured by Hoechst dye intercalation, matrix deposition was evaluated by DMMB dye. Expression of collagen II and aggrecan mRNAs was assessed by RT-PCR, followed by gel electrophoresis. In a 28-day in vitro culture, administration of 5 μg/ml CS-A, 50 μg/ml CS-B, 50 μg/ml CS-C, 5 μg/ml HS, and 500 kDa HA led to significant increase in biosynthesis rate of PGs. Gene expression of aggrecan and collagen II were upregulated by CS-A, CS-C and HA. These results showed considerable relevance of GAGs to the issue of in vitro/ex vivo neo-cartilage synthesis for tissue engineering and regenerative medical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Articular cartilage is limited to have self-repair capacity due to its avascular characteristic and inactive mitotic activity of chondrocytes. Inferior fibro-cartilaginous tissue which may induce osteoarthritis generally forms after arthroplasty, mosaicplasty, or periosteal autografts. Tissue engineering approach is currently used to prepare transplantable hyaline-like tissue which may provide a more suitable alternative to repair defects [1, 2].
Type II collagen (COL II) is the major extracellular matrix (ECM) component of cartilage, and may be processed into porous scaffold to cultivate the chondrocytes for cartilage repair [3–5]. During in vivo application, crosslinking of collagen is necessary to reduce its antigenicity, prevent enzymatic degradation, and improve mechanical properties. A number of recent studies have reported that genipin, a natural crosslinking agent, is nontoxic [6, 7] and can retard protease degradation of the collagen scaffold [4].
Chondroitin sulfates (CS-A and CS-C), dermatan sulfates (DS, also known as CS-B), heparan sulfates (HS) are present in aggrecan as the GAGs side chains, whereas aggrecan is the common type of proteoglycans (PGs) in the cartilage tissue. They play an important role in directing cell-to-cell interaction through cell surface receptors, and mediate cell–cell signaling, recognition and adhesion [8]. In particular, CS has been employed in scaffolds for engineering cartilage [9, 10]. HS has been reported to have inhibitory effects on aggrecan degradation in bovine synovium explants cultures [11], and may thus have some potential toward cartilage treatments. In addition, hyaluronic acid (HA), backbone of the PGs, has been reported potentially beneficial to cartilage development [12, 13].
The aim of this study is to investigate the effects of these GAGs when present in the culture medium on the chondrocytes grown on genipin-crosslinked COL II scaffold in a more systematic manner. Expression of key cartilage genes (COL II and aggrecan), cell proliferation rate and ECM synthesis rate were assessed for this purpose.
2 Materials and methods
2.1 Collagen isolation and matrix preparation
Native insoluble COL II was isolated from bovine trachea as previously described [5]. COL II thus made was homogenized in 0.5 M acetic acid at 4°C to give a final concentration of 1.2% (wt/vol). The solution was poured into an 8-mm diameter cylindrical mold to a depth of 5 mm. The mold was placed in a −20°C freezer for 12 h and then lyophilized at −60°C for 24 h. The fabricated porous COL II matrix, each weighing ca. 3 mg, was immersed in 4.4 mM genipin (Wako, Japan) solution for 36 h for crosslinking. The matrix was rinsed with 70% (v/v) ethanol (3 times × 30 min) and PBS (5 times × 60 min).
2.2 Culturing of chondrocytes
Normal human articular chondrocytes were obtained from Cambrex BioScience (Walkersville, MD, USA). After seeding with 1 ml cell suspension (third passage, seeding density showed at Table 1, respectively) through a 24 gauge syringe into each matrix, matrix was placed in medium-filled 24-well plate in an incubator at 37°C. The matrix was transferred into a new well every other week to avoid formation of cell monolayer on the bottom of the well. The medium consisted of DMEM (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS, 10270-106, Gibco), together with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.025 μg/ml amphotericin B, 1.5 mg/ml sodium bicarbonate and 1% (v/v) ITS liquid medium supplement (Sigma, MO, USA). The culture medium was changed at 3-day intervals.
Exogenous GAGs purchased from Sigma (USA), including chondroitin sulfate A (C-9819), dermatan sulfate (C-3788), chondroitin sulfate C (C-4384), heparan sulfate (H-7640), were administered to basal medium. Two different molecular weights (500 and 40 kDa) HA were from Lifecore, USA. Effects of each GAGs on human chondrocytes as a function of time was assessed independently. Cultures were terminated at day 7, 14, and 28.
Due to the limited number of cells obtained from a particular batch of human articular chondrocytes, the experiments were conducted with cells originated from more than one batch of cells. However, results of each group (individual type of GAGs on experimental and control runs) were derived from a single batch of cells.
2.3 DNA and GAGs content
The DNA content, a measure for cell proliferation, was evaluated using the Hoechst 33258 fluorescent dye assay. Cell-seeded matrix was first cut into small pieces and digested in a papain solution composed of 55 mM sodium citrate, 150 mM sodium chloride, 5 mM cysteine hydrochloride, 5 mM EDTA and 0.56 unit/ml papain (Sigma, USA). The sample was incubated at 60°C, for 24 h. To 50 μl of cell digested solution was added 1.5 ml Hoechst dye solution (Hoechst 33258, Fluka, USA). The fluorescence of the sample was measured at 350 nm excitation and 455 nm emission. Calf thymus DNA was used as standard. The total GAGs content of each cultured construct digested by papain as described before were determined by a modified DMMB (1,9-dimethylmethylene blue) dye binding method [14]. A 40 μl of digested solution was added to 250 μl of DMMB solution at pH 1.5. Absorbance at 595 nm was measured. A standard curve was established using a series of CS concentrations.
2.4 RT-PCR analysis
To obtain information about the differentiation status of chondrocytes in the cell-seeded matrix, mRNA levels of types II and aggrecan was assessed using RT-PCR at day 28. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as standard. Cell within the matrix was extracted with TRIzol reagent (Invitrogen, USA) for RNA. A total RNA sample of 0.5 μg was used for a reverse transcriptase reaction to synthesize the first strand cDNA using BD PowerScriptTM Reverse Transcriptase (Clontech, USA). cDNA was amplificated using Taq DNA polymerase (Life Technologies). To estimate the relative mRNA levels, samples were taken at increasing cycle numbers with a two cycle interval, representing approximately a four-fold increase in mRNA. The PCR products were electrophorised in 2.0% agarose gels containing ethidium bromide. The primers were shown in Table 2.
2.5 Statistical analysis
Data from at least five independent samples were evaluated and are represented as mean ± SD. Statistical analysis was performed by one-way analysis of variance (ANOVA) and followed by multiple pair wise comparisons of the sample means using the Student’s t-test to determine the differences among the groups. The results were considered significantly different at P < 0.05.
3 Results
3.1 Effects of GAGs on cell proliferation and ECM deposition
Cell proliferation was assayed by DNA content, while ECM (more exactly PGs) deposition was estimated by the reaction of sulfated groups on PGs per unit DNA present in the sample. The results reported in Table 1. Administration with different kind and dose of exogenous GAGs as a function of time was assessed independently.
3.1.1 CS-A
The proliferation rate of 50 μg/ml CS-A was significantly lower at day 14 (P < 0.05) and day 28 (P < 0.05). After day 7, cells continued to synthesize significantly more GAGs in the presence of 5 μg/ml CS-A (day 7, P < 0.05; day 14, P < 0.05; day 28, P < 0.005).
3.1.2 CS-B
Administration of the 50 μg/ml CS-B resulted in the overall highest cell proliferation at day 28 (P < 0.005). In addition, CS-B showed that at higher dose stimulated ECM deposition significantly at day 14 (P < 0.005) and day 28 (P < 0.005). The addition of CS-B induced a further significant increase in GAGs/DNA levels within the matrix in a dose-dependent manner.
3.1.3 CS-C
When CS-C was administered, 50 μg/ml CS-C stimulated growth very significantly at day 7 (P < 0.005) and 14 (P < 0.005), but 5 μg/ml CS-C had the faster growth rate at day 28 (P < 0.005). Of matrix synthesis rate, 50 μg/ml CS-C elicited higher ECM deposition throughout the time course (day 7, P < 0.05; day 14, P < 0.05; day 28, P < 0.05).
3.1.4 HS
In case of HS, the proliferation decreased for the higher dose (50 μg/ml) run at day 28 (P < 0.05), while there was no significant difference between lower dose run and control. At a 5 μg/ml of HS, a significant increase in ECM deposition at day 7 (P < 0.05), day 14 (P < 0.05), and day 28 (P < 0.005).
3.1.5 HA
With exogenous HA administration (500 μg/ml, 40 and 500 kDa), no significant difference was detected in proliferation rate as compared to the control. However, the higher M.W. HA (500 kDa) had a much more prominent effect on enhancing the ECM deposition at day 7 (P < 0.005), 14 (P < 0.005), and 28 (P < 0.005).
3.2 Effects on the gene expression
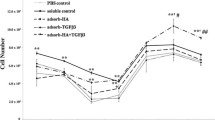
According to the GAGs/DNA results, we selected each GAGs given higher ECM deposition for further gene expression examination using RT-PCR. The experimental group studied were as follows: CS-A(5 μg/ml), CS-B(50 μg/ml), CS-C(50 μg/ml), HS(5 μg/ml), and two kinds of M.W. HA(40 and 500 kDa). After 28 days of culture, we analyzed the mRNA expression of two major cartilage-specific genes: aggrecan and collagen II. Results were displayed via gel staining images in Fig. 1a. It is obvious that both aggrecan and collagen II gene were up-regulated by exogenous GAGs administration. As for HA results, shown in Fig. 1b, indicated 500 kDa HA induced higher collagen II gene expression than the 40 kDa and control runs, while there was no significant difference in aggrecan gene expression between two kinds of M.W. HA administration but were both higher than the control.
Effects on the gene expression with selected GAGs mRNA expression as detected by RT-PCR in human chondrocytes cultured on collagen II scaffold and administration with GAGs (x-axis) for 28 days. a Control: basal medium; CS-A (5 μg/ml); CS-B (50 μg/ml); CS-C (50 μg/ml); HS (5 μg/ml). b Control: basal medium; 40 kDa: HA (40 kDa); 500 kDa: HA (500 kDa)
4 Discussion
CS (CS-A and CS-C) are ubiquitously found as components of PGs side chains in the ECM and at cell surfaces, and they are found in cartilage as a major component. The CS-PGs are thought to play roles in a wide range of biological processes, including mechanical and structural support, cell adhesion, motility, and differentiation. Investigations on the fine structure of CS have revealed patterns of sulfation affected cartilage development and maturation [15, 16]. We here reported beneficial effects of administration GAGs to cultured medium can make chondrocytes excrete ECM and enhance normal cartilage gene expression after 28 days culture, especially group CS-A (5 μg/ml) and CS-C (50 μg/ml).
Heparan sulfate contains polymorphic sulfated sequence motifs that are responsible for numerous protein binding and regulatory properties. HS chains are attached to core proteins to form HS-PGs, which are known to have diverse biological functions [17]. When HS is present, it helps regulate the main cellular activities. They are involved in biological processes such as migration, differentiation and morphogenesis [18]. By dispersing HS (5 μg/ml) to medium, environment promotes cell–cell and cell–matrix interactions may be formed. Analogous results occurred in the group of dermatan sulfate (CS-B). Decorin, a small dermatan sulfate PGs, is regularly and orthogonally arrayed at the surface of type I collagen fibrils. It is suggested that DS PGs are involved in the inhibition of the lateral growth of collagen fibrils, the inhibition of calcification, and in maintaining the structural integrity of aligned fibrils [16]. However, addition of CS-A and CS-C are apparently more effective than HS and CS-B not only in up-regulating gene expression of aggrecan and collagen II but also having a trend towards higher GAGs/DNA accumulation. It may be related to the fact that the major forms of PGs in the cartilage, aggrecan, contain CS as their main GAGs component.
HA is known to interact with chondrocytes via various surface receptors including CD44. It has been shown to influence many processes within the ECM including matrix assembly, cell proliferation, cell migration, and tissue development [19]. It has been suggested that the age-related decrease in the size of HA in cartilage reflects a modification occurring in the matrix [20]. Consequently, HA size appears to be critical to the response of chondrocytes. In our results (Fig. 1b), higher molecular weight HA can definitely stimulate the gene expression of collagen II and biosynthesis rate of chondrocytes. We suppose higher molecular weight HA can assemble more easily to form the large PGs aggregation.
In conclusion, our results suggest that chondrocytes cultured on genipin-crosslinked scaffold show better differentiation tendency toward functional hyaline cartilage by administering GAGs to culture medium. This was supported by the observation of up-regulation of biosynthesis rate (GAGs/DNA ratio) and gene expression of aggrecan and collagen II in vitro. Particularly, we showed that treatment with CS-A, CS-C, and 500 kDa HA led to a significant up-regulation of collagen II and aggrecan expression. These results suggest that exogenous GAGs administration are capable of activating normal cartilage ECM secretion and may find some potential applications in cartilage treatment.
References
Banu N, Tsuchiya T. Markedly different effects of hyaluronic acid, chondroitin sulfate-A on the differentiation of human articular chondrocytes in micromass, 3-D honeycomb rotation cultures. J Biomed Mater Res. 2007;80A:257–67.
Zhao HG, Ma L, Gong YH, Gao CY, Shen JC. A polylactide/fibrin gel composite scaffold for cartilage tissue engineering: fabrication and an in vitro evaluation. J Mater Sci Mater Med. 2009;20:135–43.
Ko CS, Wu CH, Huang HH, Chu IM. Genipin cross-linking of type II collagen-chondroitin sulfate-hyaluronan scaffold for articular cartilage therapy. J Med Biol Eng. 2007;27:7–14.
Ko CS, Huang JP, Huang CW, Chu IM, Type II. collagen-chondroitin sulfate-hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering. J Biosci Bioeng. 2009;107:177–82.
Pieper JS, van der Kraan PM, Hafmans T, Kamp J, Buma P, van Susante JLC, et al. Crosslinked type II collagen matrices: preparation, characterization, and potential for cartilage engineering. Biomaterials. 2002;23:3183–92.
Sung HW, Chen CN, Huang RN, Hsu JC, Chang WH. In vitro surface characterization of a biological patch fixed with a naturally occurring cross-linking agent. Biomaterials. 2000;21:1353–62.
Sung HW, Huang RN, Huang LLH, Tsai CC. In vitro evaluation of cytotoxicity of a naturally occurring crosslinking reagent for biological tissue fixation. J Biomater Sci Polym Ed. 1999;10:63–78.
Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mole Cell Biol. 2003;4:926–37.
Park SN, Park JC, Kim HO, Song MJ, Suh H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide cross-linking. Biomaterials. 2002;23:1205–12.
Pieper JS, Oosterhof A, Dijkstra PJ, Veerkamp JH, van Kuppevelt TH. Preparation and characterization of porous cross-linked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials. 1999;20:847–58.
Vankemmelbeke MN, Holen I, Wilson AG, Ilic MZ, Handley CJ, Kelner GS, et al. Expression, activity of ADAMTS-5 in synovium. Eur J Biochem. 2001;268:1259–68.
Barbucci R, Lamponi S, Borzacchiello A, Ambrosio A, Fini M, Torricelli P, et al. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials. 2002;23:4503–13.
Fioravanti A, Cantarini L, Chellini F, Manca D, Paccagnini E, Marcolongo R, et al. Effect of hyaluronic acid (MW 500–730 kDa) on proteoglycan and nitric oxide production in human osteoarthritic chondrocyte cultures exposed to hydrostatic pressure. OsteoArthr Cartil. 2005;13:688–96.
Enobakhare BO, Bader DL, Lee DA. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1, 9-dimethylmethylene blue. Anal Biochem. 1996;243:89–91.
Lauder RM, Huckerby TN, Brown GM, Bayliss MT, Nieduszynski IA. Age-related changes in the sulphation of the chondroitin sulphate linkage region from human articular cartilage aggrecan. J Biochem. 2001;358:523–8.
Sugahara K, Mikami T, Uyama T, Mizuguchiz S, Nomuraz K, Kitagawa H. Recent advances in the structural biology of chondroitin sulfate, dermatan sulfate. Curr Opin Struct Biol. 2003;13:612–20.
Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77.
Yip GW, Ferretti P, Copp AJ. Heparan sulphate proteoglycans, spinal neurulation in the mouse embryo. Development. 2002;129:2109–19.
Allemann F, Mizuno S, Eid K, Yates KE, Zaleske D, Glowacki J. Effects of hyaluronan on engineered articular cartilage extracellular matrix gene expression in 3-dimensional collagen scaffolds. J Biomed Mater Res. 2001;55:13–9.
Kamada H, Masuda K, Souza ALD, Lenz ME, Pietryla D, Otten L, et al. Age-related differences in the accumulation and size of hyaluronan in alginate culture. Arch Biochem Biophy. 2002;408:192–9.
Acknowledgment
This research is supported by Ministry of Economic Affairs, Taiwan (Technology Development Program for Academia 91-EC-17-A-17-S1-0009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, CH., Ko, CS., Huang, JW. et al. Effects of exogenous glycosaminoglycans on human chondrocytes cultivated on type II collagen scaffolds. J Mater Sci: Mater Med 21, 725–729 (2010). https://doi.org/10.1007/s10856-009-3889-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-009-3889-8