Abstract
In order to improve the mechanical properties and control the degradation rate of hyaluronic acid (HA) an investigation of the structural and mechanical properties of the hydrogels crosslinked using divinyl sulfone (DVS), glutaraldehyde (GTA) and freeze-thawing, or autocrosslinking has been carried out. The thermal and mechanical properties of the gels were characterised by differential scanning calorimetry (DSC), dynamic mechanical thermal analysis (DMTA) and compression tests. The solution degradation products of each system have been analysed using size exclusion chromatography (SEC) and the Zimm–Stockmayer theory applied. Autocrosslinked gels swell the most quickly, whereas the GTA crosslinked gels swell most slowly. The stability of the autocrosslinked gels improves with a reduction in solution pH, but is still poor. GTA and DVS crosslinked gels are robust and elastic when water swollen, with glass transition values around 20°C. SEC results show that the water soluble degradation products of the gels show a reduction in the radius of gyration at any particular molecular weight and this is interpreted as indicating increased hydrophobicity arising from chemical modification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hydrogels based on hyaluronic acid (HA) or hyaluron have gained attention as possible cell transplantation vehicles for the regeneration of a variety of tissues. The mechanical and physical properties of the gel depend on the interaction of the constituent polymer with water and generally as the specific amount of absorbed water increases, the gels permeability to oxygen and its strength decreases [1]. A high equilibrium swelling promotes nutrient diffusion into the gel and cellular waste removal out of the gel, while the insolubility provides the structural integrity necessary for tissue growth. The structural integrity of hyaluron hydrogels is determined by the crosslinks formed by chemical bonds and by physical interactions. These gels are generally biodegradable, processed easily, can be delivered in a minimally invasive manner but must have mechanical and structural properties similar to tissues and the extra cellular matrix (ECM) due to their similarity to the body’s own highly hydrated composition. It is of great interest to create hydrogels with controlled mechanical properties for biomedical applications and these must possess the mechanical strength and flexibility sufficient to withstand compressive forces from the surrounding tissues in vivo without deformation or collapse [2]. In addition, the mechanical properties of materials to which cells adhere can profoundly affect the function of the cells [3].
Hyaluronic acid a glycosaminoglycan (GAG) is a particularly attractive hydrogel material for biomedical applications [4]. GAG’s have a multifunctional role in wound healing. The early presence of a HA rich matrix enhances infiltration of migratory cells into the injured tissue and also creates an environment that promotes both cell motility and prolifereation [5]. HA also acts as a signaling molecule for cell migration and proliferation [6] and the degradation products modulate the inflammatory response and stimulate angiogenesis. For these reasons HA is an attractive building block for new biocompatible and biodegradable polymers with possible applications in drug delivery [7], tissue engineering [8–10], and visco-supplementation [11, 12]. As a polysaccharide of the extra cellular matrix (ECM), it plays a multi-task role, having many structural, rheological, physiological and biological functions in the body. It is a linear and anionic polymer consisting of two modified sugars, glucuronic acid and N-acetylglucosamine, with the molecular structure: [d glucuronic acid (1-b-3) N-acetyl-d-glucosamine (1-b-4)]n. It has a high capacity for lubrication, and water sorption and retention allowing application in ophthalmic surgery [13]. HA also plays a critical role as a signaling molecule in cell motility [14], cell differentiation [15], wound healing [16, 17]. However, poor mechanical properties and rapid degradation in solution limit broader ranges of clinical application and emphasise the need for some property improvement.
To improve the mechanical properties and control the degradation rate, HA can be chemically modified or crosslinked. Crosslinking is the most common modification of hyaluronan to form a hydrogel and a number of mechanisms have been reported in the literature [18–21]. The functional groups which are mainly responsible for crosslinking of HA molecules are the hydroxyl and carboxyl groups. Hydroxyl groups may be crosslinked via an ether linkage and carboxyl groups via an ester linkage. If desired the HA may be chemically modified prior to crosslinking to form other chemically reactive groups. Thus for example HA may be treated with acid or base such that it will undergo at least partial deacetalisation, resulting in the presence of free amino groups which can then be cross-linked via an amide (–C(O)– NH–); imino (–N=CH–) or secondary amine (–NH–CH–) bond. An imino linkage can be converted into an amine linkage in the presence of a reducing agent.
The fabrication of novel HA-based materials has been described previously [21, 22]. Here, we report a systematic investigation of the structural and mechanical, properties of the hydrogels focusing on homogeneous reactions using divinyl sulfone (DVS), glutaraldehyde (GTA) and autocrosslinked samples, it having been demonstrated that such hydrogels remain insoluble for periods of time ranging from a few hours to several days. GTA is believed to form either a hemiacetal or an ether link with HA under acidic conditions. With DVS the crosslinking occurs via the hydroxyl groups forming an ether bond. The proposed reaction schemes have been reported in a previous publication [21]. “Autocrosslinking” was also carried out and the mechanism here is postulated to occur through the development of extensive and semi-permanent secondary bonding between HA molecules. Okamoto et al. [23] maintain that during the freezing period at a low pH the electrostatic repulsive forces between the HA molecules are suppressed so the HA molecules are packed closely together to facilitate the formation of a gel. For such a gel to be stable at higher temperatures than the temperature of formation, it must be presumed that either a crystallisation process occurs, or at least a process analogous to crystallisation in which the relationship between the gelation temperature and the gel melt temperature is similar to that between Tc and Tm in conventional melt processed polymers.
The present study examines the relationships between crosslink density and mechanical properties and degradation rate and mechanism of HA hydrogels. The relationship between crosslink density and solvent swelling is described by the well known Flory–Rhener equation which demonstrates that increasing crosslinker effectiveness will be shown by a reduced volumetric swelling. For this work the swelling ratio (SR) was calculated via the equation:
where W s is the weight of the sample at equilibrium at each temperature and W d is the weight of the dried sample.
All the hydrogels were mechanically characterised by dynamic mechanical thermal analysis (DMTA) and thermally by differential scanning calorimetry (DSC). The hydrolytic degradation products of the gels were investigated using the Zimm–Stockmayer theory using size exclusion chromatography (SEC). HA is a linear molecule prior to crosslinking and if the water soluble degradation fragments are examined, their architecture and in particular their degree of branching, will give some indication of the original structure and the mechanism of breakdown.
2 Materials and methods
2.1 Materials
The sodium salt of HA with an average molecular weight of 2.06 × 106 was supplied by Clear Solutions (New York, NY) as dry powder. This material is prepared in high yield from streptococcus bacteria by fermenting the bacteria under anaerobic conditions in CO2 enriched growth medium [24]. GTA, and DVS were purchased from Lancaster (UK).
2.2 Hydrogel preparation
The crosslinking procedure has been outlined previously [25]. Briefly, in order to obtain a crosslinked HA gel with DVS, HA was dissolved in dilute alkaline solution (4 wt%) and DVS was added dropwise. Generally, one hour was enough for completion of the crosslinking reaction. All gels were optically clear, with a smooth surface. For crosslinking to proceed with GTA the aqueous HA mixture should be acidic and varying amounts of crosslinker were used. This crosslinker produced the most mechanically robust gels and they were also easily moulded into any shapes. All gels were washed to remove any unreacted crosslinker. With autocrosslinking HA aqueous (1 wt%) solutions were prepared. The pH of the solution was adjusted to the required value using 1 M HCl and was then placed in a freezer at −20°C for 2.5 days and then thawed at 25°C.
2.3 Swelling measurements
The swelling ratio (SR) of a hydrogel was measured after it was swollen to a desired state at 25°C. It was carefully taken out from the solution, wiped with a filter paper for the removal of the free water on the surface, and then weighed. SR (g/g) of a sample was calculated according to Eq. 1. All measurements were made in triplicate for each sample.
2.4 Analysis of degradation
A degrading crosslinked hydrophilic polymer can be expected to generate large soluble fragments and so characterisation of these is a useful indicative parameter of the mechanism and original structure. The soluble degradation products can be compared with the original polymer using SEC and this was done using Viscotek Model 270 chromatograph with a GMPWXL mixed bed column at 25°C. Solvent flow was maintained at 0.7 ml/min through the column. The model 270 system consisted of a dual detector, a viscometer and a light scattering detector (LALS and RALS). Both detectors are then connected with a refractive index detector (RI 2000). HA is a random coil molecule, so it has an α value between 0.5 and 1.0, or greater is possible for extended rod-like molecules. The g′ branching index is calculated as the ratio of sample [η] B value against its linear reference [η] L value of same molecular weight:
To relate the viscosity branching index g with g′, an assumption has to be made, i.e. g and g′ have a relationship as shown in Eq. 3.
where ε is the shape factor which is taken to be 0.75 assuming random branching [26].
The relation between the number of branches per molecule and the branching ratio depends on the branching functionality and the polydispersity of the sample of branched molecules. Assuming the degraded gel is a trifunctional randomly branched polymer, the Zimm–Stockmayer equation is:
where B w is the weight average number of branches per molecule of a polydisperse sample.
The branching frequency is calculated as follows:
where R is the repeat unit, M w is the molecular weight. A repeat unit of 37800 was used for these calculations, this value is equivalent to 100 HA units [26–28].
2.5 Characterisation of hydrogels
Differential scanning calorimetry analysis was carried out using a TA Instruments DSC 10 differential scanning calorimeter. The samples were hermetically sealed within the DSC pans. The thermal analysis profiles were of water swollen samples and the temperature was increased from ambient to 300°C at a rate of 10°C /min under 60 cc/min of nitrogen gas flow. Samples were tested in triplicate to ensure reproducibility. The DMTA of the materials was carried out with a Polymer Laboratories DMTA MK-1 apparatus, operating in the parallel plate mode. The scans were performed on samples maintained under room conditions, at a frequency of 1 Hz, temperature range of −30 to 100°C, and a heating rate of 4°C min−1. Multi-frequency tests were carried out on the DVS crosslinked gels.
The more robust HA gels crosslinked by GTA were compression tested and Fig. 1 shows a typical sample used for this. A compression rig was set up on an Instron 4302 machine using 100 N load cell with a crosshead speed of 5 mm/min. Samples were compressed to 50% of their initial thickness and the swollen modulus, G e, of each sample was calculated using Eq. 6 since uniaxial compression measurements were carried out on the hydrogels between two parallel plates [29].
where F is the force, A is the original cross sectional area of the swollen hydrogel, and λ = l/l o where l and l o are the lengths of the gel after and before compression, respectively. Plotting F/A versus λ − λ−2 resulted in a straight line with a slope of G e, which was the modulus of elasticity of the swollen hydrogel.
Cyclical compressive testing between a compressive strain of 0.2 and 0.4 at a crosshead speed of 1.25 mm/min was used to evaluate sample resilience and hysteresis. All tests were performed at room temperature.
3 Results and discussion
3.1 Swelling studies
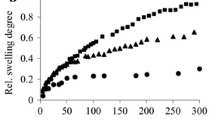
Figure 2 shows the short term swelling behaviour of the DVS, GTA and pH 1.5 autocrosslinked gels at 25°C in distilled water and Fig. 3 shows the longer term behaviour of the DVS crosslinked gels. Figure 4 compares the swelling behaviour of three autocrosslinked gels prepared at pH values of 2.0, 7.0 and 9.0.
The speed at which the gels swell to their equilibrium water content is presumed to be a function of crosslink density and hydrophobicity of the system, and comparison of the results for the three different crosslinking methods suggests that GTA is the most effective crosslinker with the autocrosslinking technique the least effective. Both of the covalently crosslinked gels are at their equilibrium swelling ratio after 6 h in distilled water and are stable beyond 24 h whereas the autocrosslinked samples have dispersed into solution before this time is reached. The effect of reducing pH is in accordance with the proposals of Okamoto et al. [28] that maximum chain interaction can be obtained at low pH, but it is apparent from the results presented here that even at 25°C the forces of solvation greatly exceed the inter-molecular binding forces. The GTA crosslinked samples are the least swollen and are in turn the most dimensionally stable of the gels; observations which are consistent with a high crosslink density. During swelling of these gels, they initially turned white in colour, and then a colourless swelling front moved inwards, separating the highly swollen surface from the less swollen core of the gel and gradually the entire gel turned colourless and was swollen evenly. This colour phenomenon is presumed to be due to molecular rearrangement as the water diffuses through the material. Between 24 and 48 h the gels began to undergo additional swelling and this is presumed to indicate degradation.
3.2 Mechanical and thermal characterisation of the hydrogels
Figure 5 shows the DSC thermograms for the covalently crosslinked and autocrosslinked and slightly swollen hydrogels and Table 1 details the important peak temperatures. The two chemically crosslinked gels produced thermograms which were similar in shape and all of the samples showed the presence of a broad endothermic peak around 100°C, which is associated with the loss of moisture remaining after the initial drying procedure. The relatively smaller endotherm obtained with the autocrosslinked material may reflect lower water retention through the drying period. Significant sharp exothermic peaks were also observed for each sample at higher temperatures and crosslinking changes the relative magnitude of these two exothermic peaks. It is thought that the first of these peaks represents conversion into a less-ordered state, and the second represents thermal degradation.
Figures 6–8 show the DMTA responses of the gels investigated and Table 2 summarises some of the important results. As expected the samples show a reduction in elastic modulus with increased water content, with the height of the tan δ peak (the α transition) being reduced and the peak broadened in the more swollen samples. In all three figures the glass transition is apparent at approximately 20–25°C. Increased water content slightly lowers the glass transition temperature of the hydrogels, however the GTA crosslinked materials have a lower than expected T g and this may arise from the presence of unreacted crosslinker which is having a plasticising effect. Since the materials are crosslinked the elastic modulus in shear does not decline to near zero values and they still exhibit useful load bearing characteristics above their glass transitions and at 37°C.
Divinyl sulfone crosslinked materials were selected for frequency sweeps and Figs. 9 and 10 show results obtained using frequencies of 0.1, 1.0 and 10 Hz. As expected, the storage modulus increases with increasing frequency, but above the glass transition the effects are small. In the glass transition region, the effect of the measurement frequency is pronounced between 0.1 and 1 Hz but less so between 1 and 10 Hz. At 37°C when the modulus reaches a rubbery plateau, the modulus increases by 18% across the frequency range. The measurement frequency effect is more uniform across the frequency range above the T g and the shift to higher apparent stiffness with increasing frequency correlates with the increase in T g as indicated by the tan δ peak. The small peak on the storage modulus directly preceding the drop associated with the T g is thought to be due to molecular rearrangement as the material relieves stresses frozen in by the processing method [30].
Figure 11 shows the results of cyclic loading on a GTA crosslinked gel. A maximum stress of 20.3 KPa was required to induce an initial compressive strain of 0.2. The first compressive cycle results in a permanent deformation of around 10% with subsequent cycles showing greater relative recovery. Although significant hysteresis is evident the overall elasticity of the gel is apparent. The engineering stress versus strain curve, obtained by treating the gel as an incompressible elastic material, is shown Figure 12. The elastic modulus was calculated from the slope and found to be 24.4 KPa for a 1:2 mole ratio crosslinked gel, which compared very favourably with elastic modulii of similar glycosaminoglycans (GAGs) measured by Kirker et al. [31].
3.3 Analysis of degradation
To characterise the degradation products samples were taken from the PBS solution surrounding the degrading gel, analysed with SEC and compared with a reference linear HA of similar original molecular weight. As an example of typical results Figs. 13 and 14 show the relationships between intrinsic viscosity and molecular weight and retention volume and molecular weight for the autocrosslinked sample. From the Mark Houwink equation, the plot of the Log IV and Log M should be linear, however in Fig. 13 the slope is not constant for the degraded sample which suggests a branched structure and Fig. 14 shows that the degraded sample has a higher molecular weight than the reference sample at the same elution volume, but the difference is decreased as the molecular weight decreases. This indicates that the polymeric degradation products possess a smaller radius of gyration for a given molecular weight, and this behaviour is usually considered to be indicative of short chain branching. Applying the Zimm–Stockmayer theory gives the branching results shown in Table 3, however these results are amenable to a number of interpretations. The polymer molecule in its original solvated form assumes a rod like conformation and any crosslinking process is likely to increase the hydrophobicity of the polymer and therefore reduce the radius of gyration and increase the molecular density of the degradation products. Additionally with the chemically crosslinked materials the large molar excess of reactant, needed to achieve acceptable crosslink densities, suggests that many crosslinker molecules may be covalently bound to the polymer, but not forming effective crosslinks. However such a scenario cannot be presumed with the autocrosslinked material where although some chain degradation may be occurring, it must be presumed that the principal process is dissolution, and the reduction in the radius of gyration at any given molecular weight must be presumed to come from changes in chain conformation. It is therefore considered that the output of the Zimm–Stockmayer approach is best considered to reflect changes in the hydrophobility of the polymer following the crosslinking processes. In that sense the apparent branching values correlate well with the efficiencies of the different crosslinking techniques used.
4 Conclusions
Comparing autocrosslinking with the use of covalent crosslinkers, then clearly autocrosslinking is attractive in that it avoids the need for reactants which may present problems of residues, and should lead to gels with the same tissue response as natural HA. However the stability of the resulting gels is poor, even when prepared at low pH. If autocrosslinking functions through crystallisation, or at least through the development of semi-ordered quasi-crystal structures, then the use of a very slow cooling rate may be beneficial in giving longer times for the polymer molecules to diffuse into the appropriate conformations relative to each other, however the results presented here for the technique are not encouraging.
Gels produced using GTA and divnyl sulphone show much higher stability having useful lifetimes of several days. Once swollen these gels are dimensionally stable, are mechanically resilient and have 37°C modulus values comparable with soft tissue.
Considering the gel degradation products the size exclusion results show a reduction in the radius of gyration at any particular molecular weight and this can be interpreted as indicating short chain branching or increased hydrophobicity with the latter explanation being considered the most likely. The inverse correlation between speed of swelling of the gel in water and the molecular density measured using size exclusion chromatograpy supports this interpretation.
References
A. Lowman, N. Peppas, Hydrogels, in Encyclopedia of Controlled Drug Delivery, ed. by E. Mathiowitz (New York, Wiley, 1999), pp. 397–418
J.H. deGoot et al., Meniscal tissue regeneration in porous 50/50 copoly(l-lactide/caprolactone) implants. Biomaterials 18(8):613–622 (1997)
D. Ingber et al., Chapter 2. in Physical Forces and the Mammalian Cell. (Academic Press, New York, 1993)
L. Lapcik, et al., Hyaluronan: preparation, styructure, properties and applications. Chem. Rev. 98(8), 2663–2684 (1998)
W.Y. Chen, G. Abatangelo, Functions of hyaluronan in wound repair. Wound Repair Regen. 7, 79–89 (1999)
E.A. Turley, P.W. Nobel, Signalling properties of hyaluronan receptors. J. Biol. Chem. 277, 4589–4592 (2002)
F.S. Palumbo, et al., New graft copolymers of hyaluronic acid and polylactic acid: synthesis and characterization. Carbohydr. Polym. 66(3), 379–385 (2006)
S.-N. Park et al., Biological characterization of EDC-crosslinked collagen–hyaluronic acid matrix in dermal tissue restoration. Biomaterials 24(9), 1631–1641 (2003)
J. Leach, et al., Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 82(5), 578–589 (2003)
T.C. Laurent (ed.), The Chemistry, Biology and Medical Applcations of Hyaluronan and its Derivatives. Wenner-Gren International Series, vol. 72 (Portland Press Ltd, London, 1998)
J.C. Fernandez Lopez, A. Ruano-Ravina, Efficacy and safety of intraarticular hyaluronic acid in the treatment of hip osteoarthritis: a systematic review. Osteoarth. Cart. 14(12), 1306–1311 (2006)
R. Barbucci et al., Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials 23(23), 4503–4513 (2002)
E. Balazs, in Sodium Hyaluronate and Viscosurgery. Meds. Healon (Sodium Hyaluronate). A Guide to Its Use in Ophthalmic Surgery, ed. by D. Miller, R. Stegmann (Wiley, New York, 1983), pp. 5–28
E. Turley, RHAMM, a Member of the Hyaladherins. in The Science of Hyaluronan Today. 1999 http://www.glycoforum.gr.jp/index.htm
L. Huang, et al., Engineered collagen-PEO nanofibrils and fabrics. J. Biomat. Sci.: Polym. Ed. 12, 979–993 (2001)
O. Oksala, et al., Expression of proteoglycans and hyaluronan during wound healing. J. Histochem. Cytochem. 43(2), 125–135 (1995)
K. Fukuda, et al., Hyaluronic acid inhibits interleukin-1-induced superoxide anion in bovine chondrocytes. Inflamm. Res. 46(3), 114–117 (1997)
K. Tomihata, Y. Ikada, Crosslinking of hyaluronic acid with glutaraldehyde. J. Polym. Sci. A: Polym. Chem. 35, 3553–3559 (1997)
K. Tomihata, Y. Ikada, Preparation of crosslinked hyaluronic acid films of low water content. Biomaterials 18(3), 189–195 (1997)
K. Tomihata, Y. Ikada, Crosslinking of hyaluronic acid with water soluble carbodiimide. J. Biomed. Mater. Res. 37, 243–251 (1997)
M.N. Collins, C. Birkinshaw, Comparison of the effectiveness of four different crosslinking agents with hyaluronic acid hydrogel films for tissue-culture applications. J. Appl. Polym. Sci. 104, 3183–3191 (2007)
M.N. Collins, C. Birkinshaw, Investigation of the swelling behaviour of crosslinked hyaluronic acid films and hydro-gels produced using homogeneous reactions. J. Appl. Polym. Sci. 109, 923–931 (2008)
A. Okamoto, T. Miyoshi, A Biocompatible gel of Hyaluronan. in Hyaluronan, ed. by J. Kennedy, G. Phillips, P. Williams (Woodhead Publishing Limited, Cambridge, 2002)
J. Bracke, K. Thacker, Hyaluronic Acid from Bacterial Culture (Diagnostic Inc, Minneapolis, MN, USA, 1985)
J.W. Burns, S. Cox, A. Walts, In United States Patent 5,017,229 (Genzyme Corporation, Cambridge, MA, USA, 1991)
J. Roovers, Branched Polymers, in Encylopedia of Polymer Science & Eng., vol. 2 (John Wiley, 1985), pp. 478–499
S. Grcev, P. Schoenmakers, P. Iedema, Determination of molecular weight and size distribution and branching characteristics of PVAc by means of size exclusion chromatography/multi-angle laser light scattering (SEC/MALLS). Polymer 45, 39–48 (2004)
M.N. Collins, in Fabrication of Porous Scaffolds for Tissue Engineering. PhD thesis (University of Limerick, 2007)
S.A. Bencherif, A. Srinivasan, F. Horkay, J.O. Hollinger, K. Matyjaszewski, N.R. Washburn, Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 29, 1739–1749 (2008)
K.P.Menard, Dynamic Mechanical Analysis Basics: Part 2 Thermoplastic Transitions and Properties. Perkin Elmer: Application Note (1999)
K. Kirker, G. Prestwich, Physical properties of glycosaminoglycan hydrogels. J. Polym. Sci. B: Polym. Phys. 42, 4344–4356 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Collins, M.N., Birkinshaw, C. Physical properties of crosslinked hyaluronic acid hydrogels. J Mater Sci: Mater Med 19, 3335–3343 (2008). https://doi.org/10.1007/s10856-008-3476-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3476-4