Abstract
Bioactive glasses (BAGs) have been studied for decades for clinical use, and they have found many dental and orthopedic applications. BAGs have also been shown to have an antibacterial effect e.g., on some oral microorganisms. In this extensive work we show that six powdered BAGs and two sol–gel derived materials have a clear antibacterial effect on 29 clinically important bacterial species. We also incorporated a rapid and accurate flow cytometric (FCM) method to calculate and standardize the numbers of viable bacteria inoculated in the suspensions used in the tests for antibacterial activity. In all materials tested growth inhibition could be demonstrated, although the concentration and time needed for the effect varied depending on the BAG. The most effective glass was S53P4, which had a clear growth-inhibitory effect on all pathogens tested. The sol–gel derived materials CaPSiO and CaPSiO II also showed a strong antibacterial effect. In summary, BAGs were found to clearly inhibit the growth of a wide selection of bacterial species causing e.g., infections on the surfaces of prostheses in the body after implantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioactive glasses (BAGs) were first introduced in the early 1970s by Hench and his co-workers, who discovered Bioglass® 45S5, capable of forming direct chemical bonds with both hard and soft tissue [1]. In general, bioactive materials are defined as materials eliciting a specific biological response at the interface of material and tissue, resulting in the formation of a bond between them [2]. The term refers to the ability of these materials to form a bone mineral-like calcium phosphate layer on their surfaces.
The base components of most BAGs are SiO2, Na2O, CaO, and P2O5. The weight percentages of these oxides vary in different glasses [2]. In an aqueous environment, alkaline and alkaline earth ions as well as silicon ions are released from a BAG [3]. This raises pH and osmotic pressure in the vicinity of a BAG [2].
Since the invention of Bioglass® 45S5, numerous glasses and glass ceramics with different compositions have been extensively studied for clinical purposes and are nowadays gaining use in dental and orthopedic applications. It has also been shown that BAGs have an antibacterial effect on some oral microorganisms [4].
Several metallic oxide powders have also been studied for their antibacterial properties: for example ZnO and MgO have shown antibacterial activity [5], and the addition of silver oxide to a BAG has been found to potentate its antimicrobial effect against Escherichia coli and Streptococcus mutans [6]. Silver-containing glass coatings of surgical sutures have also shown promising antibacterial properties [7].
In this work we report the antibacterial efficacy of six different bioactive glass powders and two sol–gel derived materials on 29 clinically important bacteria. The exact enumeration of alive bacteria used in the inoculate is instrumental for the reliability of testing the antibacterial effects of different materials. With this aim, we introduce an accurate and rapid flow cytometric (FCM) method to evaluate the viability and concentration of the bacterial suspensions used in the antibacterial tests of BAGs.
Materials and methods
Materials
Powdered BAGS MBG0103, MBG0118, MBG0123, S53P4, 13-93, and H2-02 used in this study were produced by Process Chemistry Centre, Åbo Akademi University, Turku [8]. The sol–gel derived materials CaPSiO and CaPSiO II were produced by the Turku Biomaterials Centre, Turku, according to a method reported previously [9]. Table 1 shows the compositions of the glasses. The glass powders were sieved to a particle size of ≤45 μm.
Microorganisms and culture conditions
Table 2 lists the microorganisms and culture conditions.
Tryptone Soy Broth (TSB) and Fastidious Anaerobe Broth (FAB) were obtained from LAB M (Bury, UK). Blood agar was made from a blood agar base (Pronadisa, Madrid, Spain) supplemented with 7.5% defibrinated sheep blood. Chocolate agar was made from Columbia agar base (Oxoid, Basingstoke, UK) supplemented with 10% defibrinated sheep blood. Lactose agar was made from the blood agar base (Pronadisa) supplemented with 2 g/L Na2HPO4 · 12H2O and 15 g/L lactose.
Preparation of samples for viability testing
Bacteria were harvested from the culture broth by centrifuging at 22,000g for 5 min. Bacterial pellets were resuspended in 0.9% sodium chloride (NaCl) and washed twice with 0.9% NaCl to remove all traces of broth. Then the bacteria were suspended and diluted 100-fold in 0.9% NaCl prior to staining and FCM analyses.
Fluorescent staining of bacteria
The LIVE/DEAD® BacLight™ Bacterial Viability Kit (Molecular Probes, Eugene, OR) was used to monitor the viability of the bacteria in suspensions. The green-fluorescent nucleic acid stain SYTO 9 labels all bacteria in a population. The red-fluorescent nucleic acid stain propidium iodide (PI) penetrates only bacteria with compromised membranes, and this reduces fluorescence emitted by SYTO 9, when these two dyes are used simultaneously.
The stock solutions of 3.34 mM SYTO 9 and 20 mM PI were diluted 100-fold in 0.9% NaCl and mixed together (1:1). Dilutions were prepared daily and stored in the dark at +4 °C. In a typical experiment, a small portion of a bacterial suspension was incubated with the mixture of SYTO 9 and PI at RT for 5 min. The final dye concentrations were 0.39 μM SYTO 9 and 2.35 μM PI. For exact counting of bacteria in the samples, TruCount® tubes (Becton Dickinson, San Jose, CA) containing a known number of fluorescent microbeads were used. A known volume of the stained sample was added to a TruCount® tube, and the sample were analyzed until 1% of the microbeads were detected. Now, 1% of the sample volume was also analyzed, and the concentration of viable and total bacteria in the sample could be calculated.
Flow cytometry
FCM analysis of the stained bacterial suspensions was done using the BD FACSCalibur™ flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) equipped with a 15 mW argon ion laser (488 nm) and a red diode laser (635 nm). FACSFlow™ (Becton Dickinson) was used as sheath fluid. Red fluorescence was detected using the FL3 detector and green fluorescence using the FL1 detector. The FCM parameters were adjusted for bacterial counting. The data were analyzed using CellQuest™ software (Becton Dickinson).
Antimicrobial activity testing
The bacteria were cultured together with different BAGs to evaluate their antimicrobial activity. The dilution series of each glass was prepared in appropriate broth (Table 2) in test tubes. The final glass concentrations tested were 100, 50, 25, 12.5, and 6.25 mg/mL of broth. Powders of BAGs were first mixed and vortexed with the broth, and then 106 bacteria shown alive with FCM were added to the mixture. Bacterial cultures without added BAGs served as controls.
The viability of the bacterial suspensions incubated with different concentrations of BAGs was assessed using solid agar plates. After 24 h cultivation in broth containing BAG, 10 μL samples from the suspensions were plated. The growth of bacteria was evaluated after cultivation on agar plates at +37 °C for 16 h. (Table 2). Absence of growth on the plates was an indicator of bactericidal i.e., killing effect of a given BAG.
Results
Flow cytometry
Cell suspensions labeled with SYTO 9 and PI was analyzed using flow cytometry, and the numbers of live bacteria in the suspensions were calculated. Figure 1 shows a typical dot plot of green fluorescence and red fluorescence gated for different cell populations and fluorescing microbeads.
Flow cytometric analysis of Staphylococcus epidermidis. In the dot plot, the fluorescence intensity of the green fluorescent nucleic acid stain SYTO 9 (Vital stain) is shown in the x-direction and the fluorescence intensity of the red fluorescent nucleic acid stain PI (Viability stain) in the y-direction. The vital bacteria are shown in LR quadrant, dying bacteria with partially compromised membrane in the UR quadrant, dead bacteria with a fully compromised membrane in UL quadrant, and bacterial debris containing no or scanty amounts of nucleic acids in the LL quadrant. The TruCount beads enabling the counting of the bacteria are shown in the top right region of the dot plot
Antibacterial tests
All eight BAGs tested inhibited bacterial growth. The concentration and time needed for the effect varied depending on the BAG. The effect also varied between bacterial species, but no significant difference was seen between gram positive and gram negative bacteria.
In general, all BAGs had a growth-inhibitory effect at a concentration of 100 mg/mL. The most effective BAG was S53P4 (Fig. 2), since it inhibited the growth of all pathogens, even the most resistant. In many cases, S53P4 had an effect with lower concentrations than any other BAG. The sol–gel derived materials CaPSiO and CaPSiO II also showed good antibacterial properties, and the latter was more effective. Glasses MBG0103, MBG0118, MBG0123, 13-93, and H2-02 were less effective than the other BAGs in killing bacteria, although they usually inhibited growth at least to some extent.
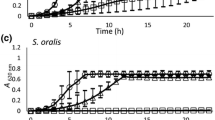
In the presence of any of the tested BAGs at a concentration of 25 mg/mL, Neisseria meningitidis totally lost its viability after 24 h and Yersinia enterocolitica after 48 h of incubation. The same phenomenon was observed with Corynebacterium ulcerans after 48 h exposure to BAG, and a strong growth-inhibitory effect was seen even at a concentration of 12.5 mg BAG/mL (Fig. 3a). Streptococcus sp. was also a group of sensitive bacteria: exposure to any BAG at a concentration of 25 mg/mL for 24–72 h resulted in total growth inhibition and 12.5 mg/mL reduced the amount of bacteria (Fig. 3b).
The most resistant species was Enterococcus faecium: during a six-day experiment none of the BAGs (100 mg/mL) could totally inhibit its growth, although exposure to BAGs reduced the number of CFUs (Fig. 3c). E. faecalis totally lost its viability only when exposed to S53P4.
All eight BAGs could inhibit or at least reduce the growth of Staphylococcus epidermidis and S. aureus. The methicillin-resistant S. aureus (clinical isolate) was killed when exposed to S53P4 and CaPSiO II.
Moraxella catarrhalis could resist the effect of most BAGs, but at a high concentration (100 mg/mL) S53P4 and CaPSiO killed it. The same was true for Klebsiella pneumoniae. But, in addition to the bactericidal effect of S53P4 and CaPSiO, CaPSiO II, and MBG0103 also had a clear inhibitory effect on K. pneumoniae (Fig. 3d).
An aliquot of 100 mg/mL of either S53P4 or CaPSiO killed Enterobacter aerogenes after 24 h and MBG0103 after 48 h of incubation, whereas 13-93 had no inhibitory effect on this species. At the same concentration glasses 13-93 and MBG0123 had only a weak effect on the growth of E. amnigenus, but it was killed in 72 h when exposed to any other BAG.
Although the time and concentration needed varied slightly, all BAGs had a bactericidal effect on Bacillus cereus, Flavobacterium meningosepticum, Haemophilus influenzae, Micrococcus sp., Pasteurella multocida, Proteus mirabilis, and Salmonella typhimurium. Listeria monocytogenes and Shigella sonnei lost their viability in the presence of CaPSiO II, S53P4, and 13-93. The growth of Escherichia coli was totally inhibited by all BAGs with the exception of H2-02, which had only a weak inhibitory effect. Glasses H2-02 and 13-93 had a bacteriostatic effect on Pseudomonas aeruginosa, whereas the other BAGs were bactericidal.
Discussion
We examined the antibacterial effects of six different BAGs and two sol–gel derived materials. About 29 clinically important bacterial species were cultivated in broth together with five concentrations of the BAGs. To our knowledge, this is the first study comparing the effects of several BAGs on a large panel of bacterial species. Even though the antibacterial effect was found to vary between bacterial species, different glass compositions, and glass concentrations, all materials tested inhibited the growth of bacteria at least to some extent. This finding was expected, since BAGs usually release ions such as sodium, calcium, phosphate, and silicate in aqueous conditions [3], and their release elevates the pH and osmotic pressure of the environment. The optimal pH of all the bacteria tested is close to neutral. Thus, the increase in pH (M. Vaahtio, unpublished data) could partly explain the growth inhibition. Another factor may be the high concentrations of calcium and alkalis likely to be released from the BAG that could cause perturbations of the membrane potential of bacteria.
Glass S53P4 had antibacterial effects at lower concentrations than the other materials tested. This glass has earlier been proven to have an antimicrobial effect on periodontal and oral bacteria [4] and also on Candida albicans [10, 11]. Other effective BAGs in this study, i.e., the sol–gel derived materials CaPSiO and CaPSiO II, have a high content of calcium oxide, which has previously been reported to potentate the antibacterial effect of metallic oxide powders [12]. Sol–gel derived materials are porous [9]. The antibacterial mechanism may also involve oxygen radicals, as earlier suggested [5]. However, the exact mechanisms of the antibacterial action of these materials still remain unknown.
It has been shown that certain supra- and subgingival bacteria have reduced viability following exposure to Bioglass 45S5® [13], and the same phenomenon was later observed with the biofilms of S. sanguis grown on Bioglass 45S5® [14]. Our results are consistent with these findings, even though we used BAGs in relatively low concentrations compared to previous studies [4, 13]. In this study, the sol–gel derived materials also had a good antibacterial effect. This is in contrast with the results of Catauro et al. [6], who showed that a sol–gel material had antibacterial properties only if it contained silver ions. The concentration of the bioactive material in their study was about the same as in ours, but its chemical composition was probably different from ours.
Practically all systems used to test the antibacterial effects of various bioactive substances have been sensitive to the inoculate effect. The amounts of live bacterial cells exposed to the materials studied have been variable, which has distorted the results. Methods based on the measurements of opacity or microscopic counting do not give accurate information on the amount of viable bacteria. On the other hand, plating and other cultivation-based methods are too slow and work-consuming for the quantification of viable bacteria for antibacterial efficacy test standardization purposes. The cytometric version of the viability determination method (BacLight™) used in the current study is based on non-selective and selective cell intrusion properties of the DNA-stains SYTO 9 and PI, respectively. We supplemented the viability determination method with an accurate counting procedure based on the use of reference beads (TruCount™). These approaches rely on the principles of cytometric bacterial counting published previously [15]. Selective staining has also been found to be a reliable tool in both viable and total counting of bacteria [16]. The discrimination between live and dead bacteria showed to be clear and the method proved to be suitable for the standardization purposes. We found that up to 20% of bacteria incubated overnight may be dead and the ratio between dead and alive bacteria varies between bacterial species. With the use of this cytometric approach, we could overcome the inoculate effect and standardize the amounts of live bacteria at each antibacterial efficacy test. The combination of viability determination and accurate FCM counting showed to be a rapid and reliable method for both quantitative and qualitative standardization of bacterial stocks used in the antimicrobial efficacy testing of BAGs.
We studied several bacterial species that can cause infections on the surface of prostheses and other artificial devices introduced in the body. These results can be used e.g., to develop antibacterial surface coatings for these devices to help prevent the tissues around them from being infected and thus, prevent the rejection of devices and further spread of the infection. A corresponding study of the effects of BAGs on the viability of anaerobic bacteria is in progress.
Conclusions
In conclusion, we showed that several BAGs have a killing effect on a large panel of clinically important aerobic bacterial species. The tested BAGs affected both gram positive and gram negative bacteria, although bacterial species specific variations were observed. Depending on the species, the concentration of BAG and the duration of exposure needed for the bactericidal effect varied.
The most effective BAG in this study was S53P4, since it inhibited the growth of all pathogens tested. In many cases, it could also inhibit bacterial growth with lower concentrations than any other BAG. Sol–gel derived materials CaPSiO and CaPSiO II also showed good antibacterial properties.
We also conclude that the antimicrobial efficacy test outcome crucially depended on the amount and vital state of inoculated bacteria. In order to standardize this biological variable we recommend that in further studies concerning the antimicrobial effects the bacterial viability determination and accurate counting should be incorporated in the efficacy test procedure.
References
L. L. HENCH and H. A. PASCHALL, J. Biomed. Mater. Res. 7 (1973) 25
L. L. HENCH and Ö. ANDERSSON, An introduction to bioceramics (Singapore: World Scientific Publishing Co, 1993) p. 41
Ö. H. ANDERSSON, J. ROSENQVIST and K. H. KARLSSON, J. Biomed. Mater. Res. 27 (1993) 941
P. STOOR, E. SÖDERLING and J. I. SALONEN, Acta Odontol. Scand. 56 (1998) 161
J. SAWAI, J. Microbiol. Methods 54 (2003) 177
M. CATAURO, M. G. RAUCCI, F. De GAETANO and A. MAROTTA, J. Mater. Sci. Mater. Med. 15 (2004) 831
J. PRATTEN, S. N. NAZHAT, J. J. BLAKER and A. R. BOCCACCINI, J. Biomater. Appl. 19 (2004) 47
H. ARSTILA, L. FRÖBERG, L. HUPA, E. VEDEL, H. YLÄNEN and M. HUPA, Glass Technol. 46 (2005) 138
T. PELTOLA, M. JOKINEN, H. RAHIALA, E. LEVÄNEN, J. B. ROSENHOLM, I. KANGASNIEMI and A. YLI-URPO, J. Biomed. Mater. Res. 44 (1999) 12
H. YLI-URPO, T. NÄRHI and E. SÖDERLING, Acta Odontol. Scand. 61 (2003) 241
M. ZEHNDER, E. SÖDERLING, J. SALONEN and T. WALTIMO, J. Endodon. 30 (2004) 220
O. YAMAMOTO, J. SAWAI, H. KOJIMA and T. SASAMOTO, J. Mater. Sci. Mater. Med. 13 (2002) 789
I. ALLAN, H. NEWMAN and M. WILSON, Biomaterials 22 (2001) 1683
I. ALLAN, H. NEWMAN and M. WILSON, Clin. Oral Implants Res. 13 (2002) 53
J. VAAHTOVUO, M. KORKEAMÄKI, E. MUNUKKA, M. K. VILJANEN and P. TOIVANEN, J. Microbiol. Methods 63 (2005) 276
L. BOULOS, M. PREVOST, B. BARBEAU, J. COALLIER and R. DESJARDINS, J. Microbiol. Methods 37 (1999) 77
Acknowledgment
This study was supported by the National Technology Agency of Finland (TEKES).
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Munukka and O. Leppäranta contributed equally to this work.
Rights and permissions
About this article
Cite this article
Munukka, E., Leppäranta, O., Korkeamäki, M. et al. Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. J Mater Sci: Mater Med 19, 27–32 (2008). https://doi.org/10.1007/s10856-007-3143-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3143-1