Abstract
Streptococcus gordonii, a bacterium involved in the initial colonization of tooth surfaces, contributes to dental biofilm formation and is an important cause of infective endocarditis. This study aimed to investigate the influence of surface reaction-type pre-reacted glass ionomer (S-PRG) filler on oral bacterial growth and aggregation of S. gordonii. The effect of various concentrations of S-PRG eluate on the growth and the biofilm formation of S. gordonii and other oral microorganisms (Streptococcus mutans, Streptococcus oralis, Lactobacillus acidophilus, and Candida albicans) was assessed. In addition, the effect of S-PRG eluate on coaggregation of S. gordonii with both S. oralis and Fusobacterium nucleatum was assessed. The effect of S-PRG eluate treatment on autoaggregation of S. gordonii was also evaluated. Our results indicate that S-PRG eluate treatment reduced both for the growth and for biofilm of all organisms in a dose-dependent manner. Coaggregation of S. gordonii with both S. oralis and F. nucleatum was inhibited by S-PRG eluate, whereas autoaggregation of S. gordonii increased at certain concentrations of S-PRG eluate. These results indicate that the S-PRG filler possesses antimicrobial activity that is mediated by inhibiting growth and biofilm of oral microorganisms, and by suppressing coaggregation of S. gordonii. In addition, these findings indicate that coaggregation of S. gordonii with other bacteria is inhibited by increased autoaggregation of S. gordonii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus gordonii and other closely related species are predominant bacteria in the human oral cavity and are known to colonize tooth surfaces by forming biofilms, which are commonly referred to as “dental plaque” [1]. S. gordonii aggregates with other oral bacteria, including other streptococci [2], actinomycetes [3, 4], and Porphyromonas gingivalis [5–7] to form biofilms. Bacterial coaggregation, which is defined as cell-to-cell adherence of different bacterial species or strains, is an integral part of plaque formation [8]. Although the cariogenicity of S. gordonii is poorly understood, the cariogenicity of S. mutans has been characterized by using specific pathogen-free rats [9]. In addition to its ability to cause oral infectious diseases, S. gordonii is known to colonize damaged heart valves and is most frequently identified as a primary etiological agent of infective endocarditis [10–12].
For preventing diseases caused by oral microorganisms, it is useful to reduce bacterial growth and to suppress oral biofilm formation. In addition to mechanical methods of maintaining oral hygiene, various other strategies such as the application of antibacterial dental materials have been developed to impede the buildup of bacteria on dental surfaces [13–15]. The antibacterial activity of composite resin containing surface reaction-type pre-reacted glass ionomer (S-PRG) filler was recently reported [16]. With pre-reacted glass ionomer (PRG) technology, a glass ionomer phase is formed on glass particles through the aqueous reaction of fluoroaluminosilicate glass with polycarboxylic acid. The S-PRG filler can be recharged with fluoride [17–20]. Various materials such as composite resin, denture base resin, pit and fissure sealants, root canal sealer, and one-step adhesive incorporate S-PRG filler [20–25].
S-PRG filler releases several types of ions (F−, Sr2+, Al3+, BO3 3−, Na+, and SiO3 2−) in distilled water and in lactic acid solution [17, 26], and composite resins with S-PRG filler exhibit antibacterial activity through release of metal ions [16]. Yoneda et al. [27] showed that S-PRG eluate inhibits the protease and gelatinase activities of P. gingivalis and suppresses coaggregation between P. gingivalis and Fusobacterium nucleatum. Another study showed that S-PRG eluate inhibits biofilm formation and disrupts mature biofilms [28]. However, the antibacterial activity of S-PRG filler has not been thoroughly assessed.
The purpose of this in vitro study was to evaluate the antimicrobial activity of S-PRG filler against the growth of S. gordonii and other oral microorganisms. In addition, the inhibitory activity of the filler against S. gordonii autoaggregation and coaggregation with other oral bacteria was also studied.
Materials and methods
Preparation of S-PRG eluate
S-PRG eluate was prepared as described previously under conditions that produce the highest concentration of each ion [26–28]. Briefly, S-PRG filler (Shofu Inc., Kyoto, Japan) was mixed with an equal amount of distilled water and was shaken gently at room temperature for 24 h. The solution was centrifuged to remove filler material, and the supernatant was filter-sterilized (pore size 0.22 μm) to remove residual insoluble materials and other microbial contaminants. The obtained clear solution was used as S-PRG eluate.
Elemental analysis of the S-PRG eluate was performed using inductively coupled plasma atomic emission spectroscopy (ICP-AES; Instrument name, ICPS-8000; Shimadzu Co., Kyoto, Japan), after preparing calibration curves corresponding to each element (standard solutions of Na, Sr, B, Al, Si, and S were used; the standard curve for each element was generated using the following concentrations: 0, 0.5, 5.0, and 20.0 ppm). In addition, the fluoride ion concentration of the S-PRG eluate was measured. Total ionic strength adjustment buffer (0.5 mL; TISAB III; Thermo Fisher Scientific Inc., Waltham, MA) was added to the S-PRG eluate, and a combination fluoride electrode (Orion 9609BN; Thermo Scientific Inc.) that was connected to a fluoride ion meter (720A; Thermo Scientific Inc.) was inserted into the sample solution to measure the released fluoride. The pH values were measured using a pH meter (pH METER F-22; Horiba Ltd., Kyoto, Japan). Before commencing pH measurement, pH electrodes were calibrated with standard solutions at pH 6.86 and pH 4.01 (Horiba Ltd.).
Bacterial growth assessment
The effect of S-PRG eluate treatment on the growth of the following organisms was assessed: S. gordonii DL1; S. mutans MT8148 and Lactobacillus acidophilus NDU121 (known as causative agents of tooth decay [29, 30]); S. oralis 34 (an early colonizer of the oral cavity and a causative agent of infective endocarditis [31, 32]); and Candida albicans NDU221 (causative agent of candidiasis [33, 34]). The two NDU strains were clinically isolated from human oral mucosa under the approval of the Ethics Committee for the Nippon Dental University School of Life Dentistry at Tokyo (approval number NDU-T2013-03) and checked to possess typical biological properties. Organisms were cultured in brain heart infusion (BHI) broth (Becton, Dickinson and Company, Sparks, MD) at 37 °C for 18 h.
Test cultures were grown under three test conditions to evaluate the effects of S-PRG eluate on bacterial growth: 1 × BHI (control, 4 mL of 1 × BHI); 1 × BHI with 20 % S-PRG eluate (2 mL of 2 × BHI, 0.8 mL of S-PRG eluate, and 1.2 mL of sterilized distilled water); and 1 × BHI with 50 % S-PRG eluate (2 mL of 2 × BHI and 2 mL of S-PRG eluate). The bacterial inoculum was obtained by growing the cultures at 37 °C for 18 h. The test cultures were set-up by inoculating the inoculum bacteria in BHI to get a final bacterial optical density (OD) of 0.01 (A 620nm) with a final culture volume of 4 mL. Bacterial cell growth in static culture at 37 °C was automatically recorded at A 650nm using a TVS062CA Bio-photorecorder (Advantec, Tokyo, Japan). Three independent experiments were performed for each assay.
Biofilm assays
Biofilm formation was assessed as described previously [35] with some modifications. The biofilm medium (BM) contained 58 mM K2HPO4, 15 mM KH2PO4, 10 mM (NH4)2SO4, 35 mM NaCl, 0.8 % glucose, 0.2 % casamino acids, and 0.05 mM MnCl2·4H2O and was supplemented with filter-sterilized vitamins (0.04 mM nicotinic acid, 0.1 mM pyridoxine HCl, 0.01 mM pantothenic acid, 1 mM riboflavin, 0.3 mM thiamin HCl, and 0.05 mM D-biotin), amino acids (4 mM l-glutamic acid, 1 mM l-arginine HCl, 1.3 mM l-cysteine HCl, and 0.1 mM l-tryptophan), and 2 mM MgSO2·7H2O. Round-bottomed polystyrene microtiter plates (Falcon®, NY, USA) containing 200 mL of test culture per well were inoculated with an 18-h bacterial culture (final A 595nm = 0.01) under four test conditions: 1 × BM (control); 1 × BM with 5 % S-PRG eluate; 1 × BM with 20 % S-PRG eluate; and 1 × BM with 50 % S-PRG eluate. Six independent experiments were performed for each assay. After 16 h of incubation at 37 °C under anaerobic conditions with agitation, 25 µL of 0.2 % crystal violet (CV) solution was added to each well. After 15 min, wells were rinsed two times with 200 µL of distilled water and dried. CV-stained biofilm on the bottom of the well was photographed using a scanner, and the images were analyzed using ImageJ version 1.48 software. Statistical differences in the means of optical values were evaluated by performing an unpaired t test. Differences were considered to be significant at P < 0.001.
Coaggregation assay of S. gordonii and other bacteria
Coaggregation assay was conducted according to the protocols described by Cisar et al. [36] and Kolenbrander et al. [37]. The assay was performed using two microbial combinations: (a) S. gordonii DL1and S. oralis 34, and (b) S. gordonii DL1 and F. nucleatum ATCC25586. A previous study demonstrated strong coaggregation reactions between S. gordonii DL1 and F. nucleatum ATCC25586 [38, 39]. In this study, streptococci were cultured as described above, and F. nucleatum was incubated anaerobically in BHI broth at 37 °C for 2–3 days. After incubation, cultures were centrifuged for 15 min at 800×g. Bacterial cells were washed twice in coaggregation buffer [CB; 10 mM Tris, 0.15 M NaCl, 0.1 mM CaCl2, 0.1 mM MgCl2 (pH 7.8)], centrifuged for 15 min at 800×g, and then resuspended in CB to obtain an optical density of 2.0 at 620 nm. Strains to be examined for coaggregation were combined with an equal volume of a test solution (CB as a control) in a total volume of 1.0 mL. To evaluate the effects of S-PRG eluate on coaggregation, the eluate was added to the mixture at final concentrations of 5, 25, and 50 %. After mixing, suspensions were kept at room temperature for 10 min, and the degree of coaggregation in the mixed suspensions was scored using a visual scoring system (from “−” to “4+”) [36, 40] as follows: “−” indicates no change in turbidity and no evidence of coaggregates in the mixed suspensions; “1+” indicates turbid supernatant with finely dispersed coaggregates; “2+” indicates definite coaggregates that do not precipitate immediately; “3+” indicates slightly turbid supernatant with formation of large precipitating coaggregates; and “4+” indicates clear supernatant and large coaggregates that precipitate immediately. All assays were repeated at least three times. Representative results are shown with an indication of reproducibility.
Autoaggregation assay of S. gordonii
Measurement of the autoaggregation percentage of S. gordonii was evaluated as described below. S. gordonii was cultured for 18–24 h and then centrifuged for 15 min at 800×g. The pellet was washed twice in CB and resuspended in CB to obtain an optical density of 2.0 at 620 nm. The bacterial suspension (100 µL) was mixed with an equal volume of the S-PRG eluate, which was previously diluted to various concentrations (final concentrations 0–20 %). The bacteria and eluate mixture (200 µL) was incubated in each well of a 96-well microtiter plate for 30 s. The mixture was then centrifuged for 2 min at 10×g (Tabletop Centrifuge KN-70; Kubota Corp., Tokyo, Japan) and supernatant (50 µL) was gently transferred to a new 96-well flat-bottomed microtiter plate. Optical density of the solution at 650 nm was measured using the SpectraMax Plus (MDS Analytical Technologies, Sunnyvale, CA). Three independent experiments were performed for each assay. Degree of autoaggregation was calculated as a percentage using the equation:
where ODA was the optical density of the solution with 0 to 20 % S-PRG eluate, and ODB was the optical density of the control solution.
Results
Ion release and pH levels
The results of elemental analysis of S-PRG eluate were as follows: Al, 20.8 ppm; B, 1683.8 ppm; Na, 554.2 ppm; Si, 10.3 ppm; Sr, 149.6 ppm; and F, 137.0 ppm. pH of the eluate was 7.7; all experiments were performed using this single batch. No pH changes or precipitation was observed after the addition of eluate to the reaction mixtures.
S-PRG eluate reduces bacterial growth
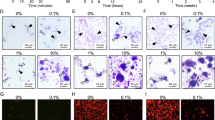
Bacterial cell growth rates of each organism after treatment with S-PRG eluate are shown in Fig. 1. The growth rate of the untreated control culture was considerably higher than those of cultures treated with S-PRG eluate. In addition, the growth inhibition occurred in a dose-dependent manner. Remarkably, growth of C. albicans was completely inhibited in culture containing S-PRG eluate (Fig. 1e).
Effect of S-PRG eluate treatment on the growth rates of various oral bacteria. Open circles, open triangles, and open squares indicate 1 × BHI (control), 1 × BHI with 20 % S-PRG eluate, and 1 × BHI with 50 % S-PRG eluate, respectively. The assays were performed using a Streptococcus gordonii DL1, b S. mutans MT8148, c S. oralis 34, d Lactobacillus acidophilus NDU121, and e Candida albicans NDU221. Data represent mean values and the whiskers indicate standard deviation (n = 3)
S-PRG eluate reduces biofilm formation
The optical values of CV-stained biofilm formation of each streptococcus species after treatment with S-PRG eluate are shown in Fig. 2. The S. gordonii biofilm formation on polystyrene surface was significantly reduced in BM broth containing S-PRG eluate (Fig. 2a). For S. mutans and S. oralis, the optical values of CV-stained biofilms cultured in media containing 25 % S-PRG eluate and 50 % S-PRG eluate were significantly lower than those of the control cultures (p < 0.001, Fig. 2b, c). In contrast to that observed with streptococci cultures, no biofilm formation was observed with L. acidophilus and C. albicans control cultures (Fig. 2d).
Effect of S-PRG eluate treatment on biofilm formation of various oral streptococci. Mean (n = 6) and SD of the optical values of CV-stained biofilms are indicated. The asterisk indicates that the optical values were significantly less than that of the control (0 % S-PRG eluate; P < 0.001). The assays were performed using a S. gordonii DL1, b S. mutans MT8148, and c S. oralis 34. Biofilm of each bacterial culture grown in the presence of various concentrations of S-PRG eluate was stained with crystal violet (d)
Effects of S-PRG eluate on coaggregation
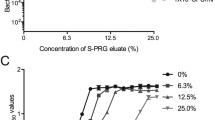
Profiles of coaggregation between S. gordonii and S. oralis or F. nucleatum in the presence or absence of S-PRG eluate are shown in Fig. 3. For the assay using S. gordonii and S. oralis, coaggregation scores of “2+,” “1+,” “−,” and “−” were obtained after treatment with 0, 5, 25, and 50 % S-PRG eluate, respectively. For the assay using S. gordonii and F. nucleatum, coaggregation scores of “4+,” “4+,” “3+,” and “3+” were obtained after treatment with 0, 5, 25, and 50 % S-PRG eluate, respectively. The formation of precipitating coaggregates was reduced only by certain concentrations of S-PRG eluate.
Effects of S-PRG eluate on autoaggregation
Degree of autoaggregation of S. gordonii in the presence of S-PRG eluate was assessed and is shown in Fig. 4. The degree of autoaggregation was low at S-PRG eluate concentrations of 8 % or lower. However, treatment with eluate concentrations of 10–16 % showed a steep increase in the degree of autoaggregation with increasing concentrations of the eluate. Notably, treatment with 18 % S-PRG eluate resulted in degree of autoaggregation that was higher than 80 %.
Discussion
The elemental analysis of S-PRG eluate showed that it contained considerably high amounts of BO3 3−, and relatively high amounts of F−, Sr2+, Al3+, Na+, and SiO3 2−. Although the mechanisms of ion release from S-PRG filler are not completely understood, it is believed that the presence of a glass ionomer phase around the glass core of the filler is related to ion release [19]. Among the ions detected in this study, boron and fluoride ions released from S-PRG filler may possess antibacterial activity [41]. In this study, the bacterial growth rates were lower in the presence of S-PRG eluate and the growth inhibition was dose dependent. In addition, inhibitory effect of S-PRG on the biofilm formation of oral streptococci was observed. It is possible that the ions released from S-PRG filler contribute to the inhibition of bacterial cell growth and biofilm formation. Interestingly, the growth of C. albicans was strongly inhibited in the culture containing S-PRG eluate. This indicates that denture base resins containing S-PRG filler could suppress oral candidiasis.
Coaggregation is thought to be important in the development of oral biofilms, as the ability of cells to bind to tooth-associated biofilm affords an opportunity to join the developing microbial community [42]. S. gordonii surface proteins, Hsa, and SspA/SspB, are known to adhere to salivary proteins and mediate coaggregation with other bacteria [43–45]. In this study, coaggregation between S. gordonii and other bacteria was inhibited depending on the concentration of S-PRG eluate. The formation of precipitating coaggregates was suspected to be inhibited by inactivation of the bacterial cell surface layer protein by the ions released from S-PRG eluate; however, further analysis of these properties needs to be carried out. In addition, a previous study has shown that the S. gordonii surface protein Hsa mediates binding of the organism to saliva-coated hydroxyapatite [46]. Thus, the utilization of S-PRG in dental materials could be useful in preventing bacterial biofilm development.
Autoaggregation is defined as the adherence of bacteria belonging to the same strain [47]. In a previous study, prsA mutants of S. mutans displayed an altered cell wall protein profile that led to increased autoaggregation, reduced resistance to mechanical breakage, and early biofilm formation [48]. Although the autoaggregation of S. gordonii has not been reported previously, autoaggregated bacteria may attenuate the increase in coaggregation with other bacteria and may be easily removed from the pellicle by brushing. In the present study, autoaggregation of S. gordonii increased depending on the concentration of S-PRG eluate used. Autoaggregation is thought to be influenced by positive ions (Sr2+, Al3+, and Na+) released from S-PRG filler, because bacterial cell surfaces are negatively charged due to the dissociation of carboxyl and phosphate groups under neutral conditions. In our previous studies, we measured the amounts of various ions released from a specimen of sealant containing S-PRG filler (internal diameter, 15 mm; height, 1 mm) into 5 mL distilled water. The levels of F−, Sr2+, BO3 3−, Al3+, Na+, and SiO3 2− ions released were found to be 12.60 ppm, 7.39 ppm, 6.60 ppm, 0.90 ppm, 15.01 ppm, and 1.02 ppm, respectively [17]. As the ions released from resin are in small quantities, the S-PRG filler may have limited antimicrobial efficacy and inclusion of S-PRG filler in toothpastes may have better antimicrobial effectiveness. Additional studies are needed for examining the antimicrobial activity of each ion as well as of the various combinations of ions. In addition, the optimal safe concentrations of these ions should be determined.
In conclusion, the results of our study indicate that the S-PRG filler has inhibitory activity both for microbial growth, biofilm, and aggregation of S. gordonii. Thus, dental materials containing S-PRG filler have the ability to inhibit oral biofilm formation.
References
Gibbons RJ. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984;63:378–85.
Whittaker CJ, Clemans DL, Kolenbrander PE. Insertional inactivation of an intrageneric coaggregation-relevant adhesin locus from Streptococcus gordonii DL1 (Challis). Infect Immun. 1996;64:4137–42.
Cisar JO, Sandberg AL, Abeygunawardana C, Reddy GP, Bush CA. Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology. 1995;5:655–62.
Palmer RJ Jr, Gordon SM, Cisar JO, Kolenbrander PE. Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J Bacteriol. 2003;185:3400–9.
Lamont RJ, Hersey SG, Rosan B. Characterization of the adherence of Porphyromonas gingivalis to oral streptococci. Oral Microbiol Immunol. 1992;7:193–7.
Park Y, Simionato MR, Sekiya K, Murakami Y, James D, Chen W, Hackett M, Yoshimura F, Demuth DR, Lamont RJ. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infect Immun. 2005;73:3983–9.
Love RM, McMillan MD, Park Y, Jenkinson HF. Coinvasion of dentinal tubules by Porphyromonas gingivalis and Streptococcus gordonii depends upon binding specificity of streptococcal antigen I/II adhesin. Infect Immun. 2000;68:1359–65.
Kolenbrander PE. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–56.
Tanzer JM, Baranowski LK, Rogers JD, Haase EM, Scannapieco FA. Oral colonization and cariogenicity of Streptococcus gordonii in specific pathogen-free TAN: SPFOM (OM) BR rats consuming starch or sucrose diets. Arch Oral Biol. 2001;46:323–33.
Baddour LM. Virulence factors among Gram-positive bacteria in experimental endocarditis. Infect Immun. 1994;62:2143–8.
Stinson MW, Alder S, Kumar S. Invasion and killing of human endothelial cells by viridans group streptococci. Infect Immun. 2003;71:2365–72.
Takahashi Y, Takashima E, Shimazu K, Yagishita H, Aoba T, Konishi K. Contribution of sialic acid-binding adhesin to pathogenesis of experimental endocarditis caused by Streptococcus gordonii DL1. Infect Immun. 2006;74:740–3.
Pupo YM, Farago PV, Nadal JM, Simao LC, Esmerino LA, Gomes OM, Gomes JC. Effect of a novel quaternary ammonium methacrylate polymer (QAMP) on adhesion and antibacterial properties of dental adhesives. Int J Mol Sci. 2014;15:8998–9015.
Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–9.
Cui C, Zhou XN, Chen WM. Self-etching adhesives: possible new pulp capping agents to vital pulp therapy. Front Med. 2011;5:77–9.
Saku S, Kotake H, Scougall-Vilchis RJ, Ohashi S, Hotta M, Horiuchi S, Hamada K, Asaoka K, Tanaka E, Yamamoto K. Antibacterial activity of composite resin with glass-ionomer filler particles. Dent Mater J. 2010;29:193–8.
Shimazu K, Ogata K, Karibe H. Evaluation of the ion-releasing and recharging abilities of a resin-based fissure sealant containing S-PRG filler. Dent Mater J. 2011;30:923–7.
Han L, Cv E, Li M, Niwano K, Ab N, Okamoto A, Honda N, Iwaku M. Effect of fluoride mouth rinse on fluoride releasing and recharging from aesthetic dental materials. Dent Mater J. 2002;21:285–95.
Itota T, Carrick TE, Yoshiyama M, McCabe JF. Fluoride release and recharge in giomer, compomer and resin composite. Dent Mater. 2004;20:789–95.
Kamijo K, Mukai Y, Tominaga T, Iwaya I, Fujino F, Hirata Y, Teranaka T. Fluoride release and recharge characteristics of denture base resins containing surface pre-reacted glass-ionomer filler. Dent Mater J. 2009;28:227–33.
Han L, Okamoto A, Fukushima M, Okiji T. Evaluation of a new fluoride-releasing one-step adhesive. Dent Mater J. 2006;25:509–15.
Itota T, Carrick TE, Rusby S, Al-Naimi OT, Yoshiyama M, McCabe JF. Determination of fluoride ions released from resin-based dental materials using ion-selective electrode and ion chromatograph. J Dent. 2004;32:117–22.
Mukai Y, Kamijo K, Fujino F, Hirata Y, Teranaka T, ten Cate JM. Effect of denture base-resin with prereacted glass-ionomer filler on dentin demineralization. Eur J Oral Sci. 2009;117:750–4.
Mukai Y, Tomiyama K, Shiiya T, Kamijo K, Fujino F, Teranaka T. Formation of inhibition layers with a newly developed fluoride-releasing all-in-one adhesive. Dent Mater J. 2005;24:172–7.
Shimazu K, Ogata K, Karibe H. Caries-preventive effect of fissure sealant containing surface reaction-type pre-reacted glass ionomer filler and bonded by self-etching primer. J Clin Pediatr Dent. 2012;36:343–7.
Fujimoto Y, Iwasa M, Murayama R, Miyazaki M, Nagafuji A, Nakatsuka T. Detection of ions released from S-PRG fillers and their modulation effect. Dent Mater J. 2010;29:392–7.
Yoneda M, Suzuki N, Masuo Y, Fujimoto A, Iha K, Yamada K, Iwamoto T, Hirofuji T. Effect of S-PRG eluate on biofilm formation and enzyme activity of oral bacteria. Int J Dent. 2012;2012:1–6.
Suzuki N, Yoneda M, Haruna K, Masuo Y, Nishihara T, Nakanishi K, Yamada K, Fujimoto A, Hirofuji T. Effects of S-PRG eluate on oral biofilm and oral malodor. Arch Oral Biol. 2014;59:407–13.
Liljemark WF, Bloomquist C. Human oral microbial ecology and dental caries and periodontal diseases. Crit Rev Oral Biol Med. 1996;7:180–98.
Tahmourespour A, Kermanshahi RK. The effect of a probiotic strain (Lactobacillus acidophilus) on the plaque formation of oral streptococci. Bosn J Basic Med Sci. 2011;11:37–40.
Corcuera MT, Gomez-Lus ML, Gomez-Aguado F, Maestre JR, Ramos Mdel C, Alonso MJ, Prieto J. Morphological plasticity of Streptococcus oralis isolates for biofilm production, invasiveness, and architectural patterns. Arch Oral Biol. 2013;58:1584–93.
Douglas CW, Heath J, Hampton KK, Preston FE. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol. 1993;39:179–82.
Felk A, Kretschmar M, Albrecht A, Schaller M, Beinhauer S, Nichterlein T, Sanglard D, Korting HC, Schäfer W, Hube B. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect Immun. 2002;70:3689–700.
Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–49.
Loo CY, Corliss DA, Ganeshkumar N. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J Bacteriol. 2000;182:1374–82.
Cisar JO, Kolenbrander PE, McIntire FC. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979;24:742–52.
Kolenbrander PE, Andersen RN, Holdeman LV. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985;48:741–6.
Palmer RJ Jr, Gordon SM, Cisar JO, Kolenbrander PE. Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J Bacteriol. 2003;185:3400–9.
Takemoto T, Hino T, Yoshida M, Nakanishi K, Shirakawa M, Okamoto H. Characteristics of multimodal co-aggregation between Fusobacterium nucleatum and streptococci. J Periodontal Res. 1995;30:252–7.
Kinder SA, Holt SC. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989;57:3425–33.
Han L, Takenaka S, Okiji T. Evaluation of selected properties of a prototype S-PRG filler containing root canal sealer. Jpn J Conserv Dent. 2007;50:713–20.
Kolenbrander PE, Palmer RJ Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8:471–80.
Egland PG, Du LD, Kolenbrander PE. Identification of independent Streptococcus gordonii SspA and SspB functions in coaggregation with Actinomyces naeslundii. Infect Immun. 2001;69:7512–6.
Jakubovics NS, Kerrigan SW, Nobbs AH, Stromberg N, van Dolleweerd CJ, Cox DM, Kelly CG, Jenkinson HF. Functions of cell surface-anchored antigen I/II family and Hsa polypeptides in interactions of Streptococcus gordonii with host receptors. Infect Immun. 2005;73:6629–38.
Takahashi Y, Konishi K, Cisar JO, Yoshikawa M. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect Immun. 2002;70:1209–18.
Nobbs AH, Zhang Y, Khammanivong A, Herzberg MC. Streptococcus gordonii Hsa environmentally constrains competitive binding by Streptococcus sanguinis to saliva-coated hydroxyapatite. J Bacteriol. 2007;189:3106–14.
Kinder SA, Holt SC. Coaggregation between bacterial species. Methods Enzymol. 1994;236:254–70.
Guo L, Wu T, Hu W, He X, Sharma S, Webster P, Gimzewski JK, Zhou X, Lux R, Shi W. Phenotypic characterization of the foldase homologue PrsA in Streptococcus mutans. Mol Oral Microbiol. 2013;28:154–65.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research No. 26861845 from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The S-PRG filler was provided by Shofu Inc. (Kyoto, Japan); however, the sponsor of the study had no role in the study design, conduct of the study, data collection, data interpretation, or preparation of the report.
Rights and permissions
About this article
Cite this article
Shimazu, K., Oguchi, R., Takahashi, Y. et al. Effects of surface reaction-type pre-reacted glass ionomer on oral biofilm formation of Streptococcus gordonii . Odontology 104, 310–317 (2016). https://doi.org/10.1007/s10266-015-0217-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-015-0217-2