Abstract
A new approach of solid polymer electrolyte (SPE) has been synthesized by agar doped with ammonium chloride and characterized by X-ray diffraction measurements, Fourier transform infrared spectroscopy and impedance spectroscopy. The highest ionic conductivity value of 4 × 10−3 S cm−1 has been achieved at room temperature for the SPE containing a concentration of 60 mol% agar with 40 mol% of NH4Cl. The prepared biopolymer electrolytes exhibit Arrhenius behaviour and dielectric effects of the prepared samples are studied. Wagner's polarisation technique is used to find the ionic transference number, which is 0.99. Linear sweep voltammetry is used to investigate electrochemical stability. The best conductive film is used to build a battery and a fuel cell, with output voltages of 1.89 V and 541 mV, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As of late, research on solid polymer electrolytes (SPEs) has acquired gigantic interest because of their application in super capacitors, electro chromic gadgets, energy units, solid-state batteries and sensors [1,2,3,4,5]. SPEs are reasonable for working at high-temperature and having great mechanical properties. Polymers like polyethene oxide (PEO) [6], polyvinyl alcohol (PVA) [7], biopolymers like methylcellulose [8] and chitosan [9] have been considered in the advancement of SPEs. However, no solid polymer electrolyte has arrived at maximum ionic conductivity at surrounding temperatures, other than Nafion.
Agar had been broadly used in food, pharmaceutical, micro-biological, medical and other industries. The use of agar in the field of electrochemistry has also been investigated [10, 11]. As a natural biopolymer, agar is more interesting than the synthetic polymers as an electrolyte in different fields owing to its higher biocompatibility and biodegradability, which make it more environmental-friendly.
In order to accomplish a significant ionic conductivity, bio-viable and bio-degradable SPE, a novel biopolymer electrolyte with a blend of agar–agar and NH4Cl have been prepared and characterized in the current review. Among the sustainable biopolymers, agar is striking because they are galactose-rooted polysaccharides extricated from seaweed having the comparable chemical structure of rehashing unit of disaccharide. A miscellaneous combination of polysaccharides: agaropectin and agarose is called agar. The two polymers share a similar galactose-supported backbone, agaropectin is intensely changed with the acidic side-groups, like sulphate and pyruvate; charge of the agarose was neutral. Agar–agar is a direct polysaccharide comprised of rotating β-(1,3)- and α-(1,4)-connected galactose deposits [12]. The appropriateness of agar gel is an option for riveted solid polymer electrolyte and Kasem has accounted for it as a polymer electrolyte for electrochemical experiments [13]. Leones et al. [14] has found a conductivity value of 2.35 × 10–5 S cm–1 for agar-based polymer electrolyte with 1-ethyl-3-methylimidazolium acetic acid derivation structure at surrounding temperature. Ionic conductivity worth 1.1 × 10−4 S cm−1 at room temperature has been accounted for by Ellen Raphael et al. [15] for agar with acetic acid. Among the inorganic salts, ammonium salts have been accounted for as great proton givers. Ammonium-based salt is picked for this new approach since proton-conducting electrolytes have been perceived for their appropriateness in the utilization of low-energy density gadgets [16, 17]. Vijaya et al. [18] has announced a conductivity of 4.52 × 10−4 S cm−1 for biopolymer electrolyte dependent on Pectin doped with NH4Cl. Sikkanthar et al. [19] has portrayed a polymer electrolyte dependent on PAN with NH4Cl which displayed an ionic conductivity in the range 10–3 S cm−1. In view of these reports, biopolymer electrolyte with another blend of agar and NH4Cl has been prepared and characterised in the current review to accomplish high ionic conductivity.
2 Experimental details
2.1 Electrolyte preparation
Agar–agar of average molecular weight 120,000 (Condo-Forja, Madrid) and NH4Cl (spectrum) were taken for this work. By the Solution-Casting technique, different compositions of agar and NH4Cl were doped to form membranes. The mixture was magnetically stirred and poured in petri dishes. The samples were dehydrated by the hot air oven. Solvent free SPE films were collected after 48 h.
2.2 Electrolyte characterization
The membranes were studied by X-ray diffraction analysis (XRD) using Cu Kα radiation at room temperature on a Philips X' Pert PRO diffractometer. The molecular interaction and chemical composition of the membranes were analysed from the FTIR spectra obtained from SHIMADZU-IR Affinity-1 Spectrometer in the extend of 400–4000 cm−1. Impedance spectra of the prepared membranes were obtained using HIOKI 3532 LCZ meter in the frequency extend 42 Hz–1 MHz, over the temperature range 303–343 K. For determining the transference number, Wagner’s DC polarization method was employed. The electrochemical stability of the polymer membrane was decided by linear sweep voltammetry using 0.1 mV s−1 scan rate. Formation of fuel cell and solid state electrochemical cell were done by using the best conducting polymer electrolyte.
3 Results and discussions
3.1 X-ray diffraction analysis
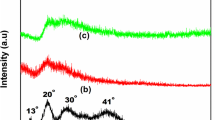
To examine the nature of agar-based films and X-ray diffraction patterns of the SPE with various compositions of NH4Cl are contemplated and are displayed in Fig. 1.
The diffractograms (Fig. 1) of agar–NH4Cl show expansive dispersed bands at the range of 2θ = 20°, 30° with a shoulder top at 2θ = 13° which have effectively been accounted by Raphael et al. [15]. It is observed that the relative intensities of these peaks decline with the expansion of NH4Cl. The broadness of the peaks increment with the increment of NH4Cl. The reduction in intensity and expansion in the width of the XRD peaks uncover that the amorphous nature of the agar–agar increments because of the addition of the dopant. It is clear from the figure that the composition of 60 mol% agar with 40 mol% NH4Cl is more amorphous. The sharp peaks at 2θ = 23°, 32.82°, 47°, 53° (JCPDS document no. 34-0710) in 50 mol% agar with 50 mol% NH4Cl are credited to NH4Cl because of the appearance of undissociated salt in the polymer lattice.
3.2 Fourier transforms infrared analysis
FTIR is a promising strategy to track down the idea of functional groups and furthermore the development of any new bonds due to doping in the SPE framework. Figure 2 shows the FTIR spectra of pure agar SPE film and agar doped with NH4Cl in various molar proportions.
From Table 1, the absorption peaks for pure agar and different pieces of agar doped with NH4Cl SPE films are shown. The wideband at 3404 cm−1 relates to the stretching of the functional compound O–H hydroxyl gathering of agar that takes part in entomb and intra-atomic hydrogen bond arrangement and has been moved slightly for all the compositions [20]. A trademark peak of the pure sample is at 934 cm−1 which is related to the in salt doped agar 3,6-anhydro galactose bridges with a minor shift [21]. The absorption band at 1370 cm−1 is because of the vibration mode of sulphate groups and it is viewed as moved to higher wavenumbers [22] in the agar doped with NH4Cl samples. The absorption peak at 1149 cm−1 is because of the vibration mode of ester-sulphate connect vibrations. The extreme pinnacle saw at 1038 cm−1 is nearly found in all polysaccharides. It is predominantly because of the coupling of the C–C or the C–O extending modes with the bending modes of C–O–H [23, 24].
The NH4Cl salt splits into two ions: NH4+ and Cl−. The NH4+ ions are made up of two identically bound hydrogen atoms, a third hydrogen atom bound more tightly, and a fourth hydrogen atom bound more weakly. The polar group of the agar polymer matrix interacts with the poorly bound H+ of NH4, which is easily dissolved by the effect of an electric field [25]. The connection between the salt with the biopolymer matrix can cause the bands to shift and the intensity of the bands to alter. The aforesaid finding supports the creation of a complex between the polymer and the salt.
3.3 AC impedance spectroscopic analysis
AC Impedance measurements are utilized to calculate ionic conductivity of the electrolyte. Figure 3a displays the Cole Cole plots of agar/NH4Cl SPE films with diverse concentrations of NH4Cl at 303 K. The plot of pure agar and 90 mol% agar:10 mol% NH4Cl comprise of a high-frequency semicircle and a spike at low-frequency. The high-frequency semicircle may possibly be the bulk properties of the polymer electrolyte [26]. In the Impedance Spectroscopic analysis, bulk resistance is measured from the Cole–Cole plot [27]. The applied field polarises the stationary polymer chains and the dominance of electrode polarisation is responsible for the low frequency spike.
a Cole–cole plot for various compositions of agar with NH4Cl at 303 K. a Pure agar b 90 mol% agar:10 mol% NH4Cl c 80 mol% agar:20 mol% NH4Cl d 70 mol% agar:30 mol% NH4Cl e 60 mol% agar:40 mol% NH4Cl f 50 mol% agar:50 mol% NH4Cl. b Variation of real part of impedance with frequency at different temperatures for 60 mol% agar:40 mol% NH4Cl polymer membrane
For other compositions, the plots show only the inclined spike suggesting that the samples are purely resistive.
Conductivity of the SPE films can be calculated from Eq. (1)
In the above equation, ‘l’ is film thickness, ‘A’ is called area between electrolyte and electrode and ‘Rb’ is denoted as a bulk resistance. The bulk resistance Rb of the SPE is attained from the end of the semicircle and the straight line inclined with the Z′-axis.
It is perceived that the 60 mol% agar:40 mol% NH4Cl composition gives high conductivity of 4 × 10−3 S cm−1. The rise in conductivity may possibly be due to large amount mobile ion concentration. The decrease in conductivity of SPE films with high salt concentration (50 mol% agar:50 mol% NH4Cl) may be due the formation of ion clusters which is evidenced from the XRD pattern. Table 2 displays, ionic conductivity of different combinations of agar with NH4Cl at various temperatures. It is seen that ionic conductivity increments with expansion in temperature. Polymer chain fragments would be enhanced at higher temperatures, resulting in an increase in absolute ionic conductivity.
Figure 3b shows the variation of real part of impedance (Z′) with frequency for the highest conductivity membrane (60 mol% agar:40 mol% NH4Cl) at different temperatures.
3.4 Conductance spectra analysis
From Fig. 4, at room temperature, the frequency relation with conductivity for various mixes of agar and NH4Cl polymer electrolytes are shown. The two regions of the plots are: a low frequency dispersion zone caused by space charge polarisation at the obstructive electrodes and a frequency independency of plateau region, which match with dc conductivity. By inferring from the plateau region to zero frequency, the dc conductivity value can be determined.
The σdc values specify a rise in conductivity with increase in frequency and it stay almost steady from definite frequency. The highest dc conductivity is observed for 60 mol% agar:40 mol% NH4Cl polymer membrane. It is also observed that dc conductivity is enhanced with the high salt concentration up to 40 mol% which is ascribed to the large number of mobile charge carriers and all ions contribute to the charge transport in the presence of external electric field. The decrease in conductivity for the agar membrane with 50 mol% of NH4Cl may be attributed to the presence of ion. Figure 5 shows the conduction spectra for the good conductivity membrane of composition 60 mol% agar:40 mol% NH4Cl at different temperatures. There are two regions in the spectra. The low frequency dispersive region and higher frequency plateau region which corresponds to DC conductivity of material. The extrapolation of plateau regions gives the value of DC conductivity. The frequency independent plateau region shifted towards the lower frequency dispersion region and the plateau region increased while increasing the salt concentration [28]. This is due to the enhancement of the available free charge carriers in the system. The rise in conductivity depends on the temperature owing to the reduction in the viscosity of the solution [29].
3.5 Temperature dependent conductivity
The conductivity for all SPE films depends on temperature which is shown in Fig. 6.
The Arrhenius equation for comparing experimental data,
where “σ”o denotes the pre-exponential factor, “Ea” denotes the activation energy, and “k” denotes the Boltzmann constant. The linear fit is revealed to be close to unity, for regression values. This shows that the prepared biopolymer membranes obey the temperature-dependent ionic conductivity and fulfils the Arrhenius relationship.
When the temperature rises, a sector's vibrational energy is sufficient to provide impetus against the force operated by its nearby atoms, resulting in a little area of space enclosing its own volume in which vibrational motion can arise. As a result, the mobility of the polymer segments increases, allowing ions to float freely along the polymer chain, thereby increases the conductivity.
Table 3 shows the activation energy obtained from log (σ) vs 1000/T graphs. The sample with the highest conductivity, 60 mol% agar:40 mol% NH4Cl, has the lowest activation energy of 0.04 eV.
3.6 Compositional dependence of conductivity
Figure 7 shows the conductivity and the activation energy as a function of different concentration of NH4Cl at room temperature.
As the conductivity of the sample increases, Ea drops, meaning that ions in highly conducting samples require less energy to migrate.
3.7 Transference number measurement
The polymer electrolytes which are responsible for the conduction have been investigated using the technique called Wagner's dc polarisation [30]. The fluctuation in polarisation current as the function of time is depicted in Fig. 8. The transference number is determined using
where ‘Ii’ and ‘If’ are the initial and final currents, respectively.
When a dc potential (1.08 V) is applied between the two aluminium electrodes sandwiching the best conductivity biopolymer electrolyte (60 mol% agar:40 mol% NH4Cl), the polarisation current is measured by the variation in time.
The total ionic transference number of 0.99 is obtained for the sample with best conductivity is 60 mol% agar:40 mol% the concentration of NH4Cl. The ionic transference number has a value near to unity. So that, the ions play a major role in charge transport in this polymer electrolyte.
3.8 Transport parameters
Using the equations below, the diffusion coefficients of anions and cations of polymer electrolyte are obtained [31].
The ionic mobility of all the samples have been determined using the equation
where ρ is density of the salt, N is the Avagadro number, e charge of the electron, k is the Boltzmann constant, T is the absolute temperature, n is the number of charge carriers, µ−, µ+ is the ionic mobility of anion and cation respectively, D−, D+ is the diffusion coefficient of anion and cation respectively.
The diffusion coefficient and ionic mobility of cations and anions calculated for all compositions of polymer membrane are shown in Table 4. The value of both diffusion coefficient (D) and mobility (μ) are increased up to the sample 60 mol% agar:40 mol% NH4Cl. The cation value of D and μ is higher value than anion in all the polymer blend electrolytes. From this, it is concluded that D and μ are responsible for the enhancement of ion conductivity in 60 mol% agar:40 mol% NH4Cl polymer blend electrolytes [32].
3.9 Dielectric spectra analysis
The complex permittivity ε* = ε′ − iε″, is commonly used to describe the dielectric response, with the real ε′ component which is attributed to charge accumulation and the imaginary ε″ component ascribes to energy loss in every cycle of the applied electric field. At 303 K, the variation in ε′ and ε″ with different compositions for agar with NH4Cl polymer electrolytes is shown in Fig. 9a, b. ε' and ε″ have very high values at low frequencies and are rather stable at higher frequencies [33]. Energy absorption or attenuation can explain the variability of ε″ with frequency. The intermittent setback of the electric field at higher frequencies is in some way that there is no surplus ion diffusion in the field direction, causing a reduction in dielectric constant. [34].
a Frequency response of dielectric constant of various compositions a 100% agar b 90 mol% agar:10 mol% NH4Cl c 80 mol% agar:20 mol% NH4Cl d 70 mol% agar:30 mol% NH4Cl e 60 mol% agar:40 mol% NH4Cl f 50 mol% agar:50 mol% NH4Cl. b Frequency response of dielectric loss of various compositions a 100% agar b 90 mol% agar:10 mol% NH4Cl c 80 mol% agar:20 mol% NH4Cl d 70 mol% agar:30 mol% NH4Cl e 60 mol% agar:40 mol% NH4Cl f 50 mol% agar:50 mol% NH4Cl
The graph of the frequency dependence of ε″ of (60 mol% agar:40 mol% NH4Cl) at room temperature shows a large frequency relaxation peak that could be done by side group dipole movement [35, 36].
3.10 Linear sweep voltammetry
The electrochemical stability is the major parameter to estimate the polymer electrolyte's stability. The maximum conducting polymer electrolyte (60% agar:40% NH4Cl) is developed to determine the polymer electrolyte's capacity to tolerate the operating voltage of a battery system in the electrochemical stability window of. A linear sweep voltammetry is using an inert stainless steel as the working electrode and the stainless steel plates as the reference and the counter electrodes. The stainless steel/60% agar:40% NH4Cl polymer electrolyte cell is built and used at a 1 mV s−1 scan rate from 0 to 5 V. The linear sweep voltammogram of the best conducting polymer electrolyte (60% agar:40% NH4Cl) as a function of voltage is shown in Fig. 10.
The breakdown voltage can be determined by the extrapolation of the straight line from the LSV curves [37]. The electrochemical stability window for the 60% agar–40% NH4Cl system is up to 3.1 V, according to the linear sweep voltammogram. As a result, this polymer electrolyte is well-suited for use in proton batteries with a working voltage of 3.1 V.
3.11 Battery characterization
In a solid state battery, the highest conductivity polymer film (60 mol% agar:40 mol% NH,4Cl) is sandwiched between two electrodes. The cathode material in this study is composed of lead oxide (PbO2), graphite, vanadium pentaoxide (V2O5),and a 60 mol% agar:40 mol% NH4Cl SPE film in the ratio of 8:2:1:0.5. Zinc powder, zinc sulphate, and graphite are used to make the anode in a 3:1:1 ratio. Thin pellets serve as the cathode and anode, respectively. The highest conductivity sample is sandwiched between the anode and prepared cathode, placed in the battery holder, which is connected with the multimeter for determining the open circuit voltage (OCV).The variation of open circuit voltage with time is shown in Fig. 11a and the charge discharge characteristics of the prepared electrochemical cell, is shown Fig. 11b. Table 5 gives the cell parameters of the prepared cell.
3.12 Construction of single PEM fuel cell
The PEM fuel cell used in this work is made up of bipolar graphite plates encased in silicon gaskets and carved with a 7.4 cm2 parallel flow channel. Two acrylic foundation plates hold these plates in place. Platinized carbon cloths with an area of 8.4 cm2 are placed above the flow channels of the either graphite plates, that act as cathode and anode, with the platinum layer acting as a catalyst. Between the electrodes, best proton conducting membrane (60 mol% agar:40 mol% NH4Cl) is sandwiched. The fuels for the single PEM fuel cell are produced by applying a 3 V voltage to a tiny electrolyser, which produces hydrogen and oxygen.
The freshly fabricated PEM fuel cell with 60 mol% agar:40 mol% NH4Cl biopolymer polymer electrolyte displayed a voltage of 541 mV and is presented in Fig. 12. The obtained voltage suggests that the biopolymer membrane with a mixture of agar and NH4Cl is a worthy contender for battery and fuel cell applications.
4 Conclusion
Novel biopolymer membrane along with agar–agar and different compositions NH4Cl is prepared by solution casting characterized by different techniques. Pattern of the XRD for agar + NH4Cl displayed the decrease in the intensity of peaks confirms the decrease in crystallinity with the rise of salt concentration. FTIR investigations confirm the complexation of NH4Cl with the agar polymer. The conductivity is increased with dopant concentration and the maximum conductivity of 4 × 10–3 is reported for 60 mol% agar:40 mol% NH4Cl. The ionic transport number measurements in the (agar:NH4Cl) polymer electrolyte suggests that ions rather than electrons are responsible for the conduction. The dielectric constant and dielectric loss are higher for the 60 mol% agar:40 mol% NH4Cl membrane, according to the dielectric analysis. The electrochemical stability window for the 60% agar–40% NH4Cl system is up to 3.1 V, according to the linear sweep voltammogram. A higher conducting film is used to create an electrochemical cell with a voltage of 1.89 V as an open circuit voltage and the parameters of the cells are reported. Similarly, single PEM fuel cell is fabricated with 60 mol% agar:40 mol% NH4Cl biopolymer electrolyte which exhibits a voltage of 541 mV.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Y.N. Sudhakar, M. Selvakumar, D.K. Bhat, Ionics (Kiel). 19, 277 (2013)
A.K. Arof, N.E.A. Shuhaimi, N.A. Alias, M.Z. Kufian, S.R. Majid, J. Solid State Electrochem. 14, 2145 (2010)
R.C. Agrawal, S.A. Hashmi, G.P. Pandey, Ionics (Kiel). 13, 295 (2007)
E. Siebert, S. Rosini, R. Bouchet, G. Vitter, Ionics (Kiel). 9, 168 (2003)
K.P. Singh, R.P. Singh, P.N. Gupta, Solid State Ionics 78, 223 (1995)
L. Ismail, S.R. Majid, A.K. Arof, Mater. Res. Innov. 13, 282 (2009)
I.S. Noor, S.R. Majid, A.K. Arof, Electrochim. Acta 102, 149 (2013)
N.E.A. Shuhaimi, S.R. Majid, A.K. Arof, Mater. Res. Innov. 13, 239 (2009)
S.R. Majid, A.K. Arof, Phys. B Condens. Matter 390, 209 (2007)
L. An, T.S. Zhao, L. Zeng, Appl. Energy 109, 67–71 (2013)
S.Y. Liew, J.C. Juan, C.W. Lai et al., Ionics 25, 1291–1301 (2019)
A.M. Stephen, G.O. Phillips, P.A. Williams, Food Polysaccharides and Their Applications (CRC Press, Boca Raton, 2016)
K.K. Kasem, J. New Mater. Electrochem. Syst. 8, 189–195 (2005)
M.M.S.R. Leones, F. Sentanin, L.C. Rodrigues, I.M. Marrucho, J.M.S.S. Esperança, A. Pawlicka, Syria Stud. 7, 37 (2015)
E. Raphael, C.O. Avellaneda, B. Manzolli, A. Pawlicka, Electrochim. Acta 55, 1455 (2010)
H. Nakajima, I. Honma, Solid State Ionics 148, 607 (2002)
R. Pratap, B. Singh, S. Chandra, J. Power Sources 161, 702 (2006)
N. Vijaya, S. Selvasekarapandian, G. Hirankumar, S. Karthikeyan, H. Nithya, C.S. Ramya, M. Prabu, Ionics (Kiel). 18, 91 (2012)
S. Sikkanthar, S. Karthikeyan, S. Selvasekarapandian, D. Arunkumar, H. Nithya, K. Junichi, Ionics (Kiel). 22, 1085 (2016)
J. Coates, Encycl. Anal. Chem. 10815 (2006).
J. Orduña-Rojas, D. Robledo, Bot. Mar. 45, 459 (2002)
P. Guerrero, A. Etxabide, I. Leceta, M. Peñalba, K. De La Caba, Carbohydr. Polym. 99, 491 (2014)
M.F.Z. Kadir, Z. Aspanut, S.R. Majid, A.K. Arof, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 78, 1068 (2011)
M.H. Buraidah, L.P. Teo, S.R. Majid, A.K. Arof, Phys. B Condens. Matter 404, 1373 (2009)
M. Hema, S. Selvasekerapandian, A. Sakunthala, D. Arunkumar, H. Nithya, Phys. B Condens. Matter 403, 2740 (2008)
S.A. Hashmi, A. Kumar, K.K. Maurya, S. Chandra, J. Phys. D. Appl. Phys. 23, 1307 (1990)
D. Vanitha, S.A. Bahadur, N. Nallamuthu, S. Athimoolam, A. Manikandan, J. Inorg. Organomet. Polym. Mater. 27, 257 (2017)
R. Sehrawat, A. Sil, Ionics (Kiel). 21, 673 (2015)
S. Das, A. Ghosh, Electrochim. Acta 171, 59 (2015)
S. Ramesh, A.H. Yahaya, A.K. Arof, Solid State Ionics 152–153, 291 (2002)
J.B. Wagner, C. Wagner, J. Chem. Phys. 26, 1597 (1957)
O.E. Geiculescu, R. Rajagopal, S.E. Creager, D.D. DesMarteau, X. Zhang, P. Fedkiw, J. Phys. Chem. B 110, 23130 (2006)
M.A. Jothi, D. Vanitha, S.A. Bahadur, N. Nallamuthu, Ionics (Kiel). 27, 225 (2021)
C.S. Ramya, S. Selvasekarapandian, G. Hirankumar, T. Savitha, P.C. Angelo, J. Non. Cryst. Solids 354, 1494 (2008)
K. Adachi, O. Urakawa, J. Non. Cryst. Solids 307–310, 667 (2002)
S. Monisha, T. Mathavan, S. Selvasekarapandian, A. MiltonFranklinBenial, G. Aristatil, N. Mani, M. Premalatha, D. Vinothpandi, Carbohydr. Polym. 157, 38 (2017)
J.C.H. Koh, Z.A. Ahmad, A.A. Mohamad, Ionics (Kiel). 18, 359 (2012)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SS. The first draft of the manuscript was written by DV and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. The first author, Dr. SLI has received the technical support from The Standard Fireworks Rajaratnam College for Women.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selvalakshmi, S., Vanitha, D., Saranya, P. et al. Structural and conductivity studies of ammonium chloride doped agar–agar biopolymer electrolytes for electrochemical devices. J Mater Sci: Mater Electron 33, 24884–24894 (2022). https://doi.org/10.1007/s10854-022-09198-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09198-2