Abstract
A system of negative temperature coefficient (NTC) thermistors with adjustable room-temperature resistivity (ρ25) and temperature sensitivity should have great application prospect for various requirements. Herein, Sb/Ga co-doped SnO2 ceramics with nominal formula of Sn0.95−xSb0.05GaxO2 (x = 0, 0.01, 0.02, 0.03, 0.04, and 0.05) were fabricated by conventional solid-state reaction process. The phase component, electrical conductivity, temperature sensitivity of resistivity and aging characteristic were investigated. Analysis of X-ray diffraction shows that the prepared SnO2-based ceramics consist of tetragonal phase. The ceramics exhibit typical characteristic of NTC of resistivity. With change of Ga-ion content, the ceramics possess adjustable ρ25 from 3.24 Ω·cm to 0.54 MΩ·cm together with related NTC material constants (B25/85 values) from 140 to 5384 K. The analysis of complex impedance spectra reveals that both band conduction and hopping conduction are responsible for electrical properties of the prepared ceramics. The aging treatment induced at 150 °C revealed that all ceramics exhibit good electrical stability. The possible aging models were investigated by analysis of the change of element valences before and after aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Typical negative temperature coefficient (NTC) thermistors exhibit the feature that the resistivity decreases exponentially with the increase of temperature, and possess the characteristics of high temperature sensitivity, good reliability, and quick response. NTC ceramic thermistors are widely applied in the regions of automobile, therapy, communication, computer, and other occasions, mainly playing a role in the suppression of surge current, overload protection, temperature measurement, and control and so on. The commonly applied materials for normal temperature NTC thermistors are the ones based on spinel transition metal oxides, such as Mn–Co–Ni–O [1], Co–Mn–O [2], Ni–Mn–O [3, 4], etc. ABO3-type perovskite compounds such as LaCrO3 [5, 6], BaTiO3 [7], and BaBiO3 [8] are also regarded as the potential candidate for NTC thermistors. On the other hand, NTC thermistors based on single-cation oxides such as SnO2, NiO, CuO, and ZnO have been recently reported for their unique feature that the room-temperature resistivity (ρ25) and NTC material constant (B25/85 value) can be effectively adjusted by changing the content and element type of solid solution ions [9,10,11,12,13]. For example, Li et al. reported that Al/La/Cu-modified ZnO-based ceramics have wide range of adjustable ρ25 and B25/85 values of 0.65–3280 kΩ·cm and 2500–5850 K, respectively [12]. Yang et al. prepared B3+/Na+-modified NiO-based ceramics with adjustable ρ25 from 47.94 Ω·cm to 1.024 MΩ·cm and B25/85 values from 2582 to 8019 K [10].
In the meanwhile, the electrical stability of NTC thermistors has recently attracted attention for its essential factor guaranteeing the reliability in practical application. Several models have been proposed to reveal the aging characteristics of NTC thermistors [14,15,16,17,18,19,20]. The change of resistance during aging treatment might be attributed to contact degradation, redistribution of cations over sublattices, and oxidation or reduction of oxygen, etc. [15]. In order to improve the aging stability, as reported by Li et al. [16], Na+ cations were added into Mn1.95Co0.21Ni0.84O4, and clustered at grain boundary to hinder the transportation of oxygen vacancies. Ren et al. investigated the aging performance of Zn–Fe–Mn–Co–O spinel-structured thermistor films at different temperatures and found that high annealing temperature can enhance the aging performance due to the improved microstructure and density of films [17]. Gao et al. studied the influence of Bi2O3 on electrical stability of Na-doped Zn0.4Ni0.6O ((Zn0.4Ni0.6)1-xNaxO) NTC ceramics, and found that Bi2O3 can effectively enhance aging stability of the thermistors [18]. The Bi2O3-rich region in ceramics provides electrons for oxygen molecules that were adsorbed by ceramic during aging process, and depresses the redox reaction of the ceramics and aging deterioration.
SnO2-based ceramics show potential candidate of NTC thermistor for their obvious advantage with effectively adjustable ρ25 and B25/85 value in wide range by changing the dopant content, but the understanding of aging characteristics of SnO2-based NTC ceramics is still insufficient. In present work, Sb/Ga co-doped SnO2 ceramics were fabricated for NTC thermistors, the related electrical properties and aging characteristic were investigated. Combined with XPS analysis, moisture adsorption and related redox reaction were proposed for the mechanism of the aging-induced resistance shift of the SnO2 ceramics.
2 Experimental
2.1 Sample preparation
According to the reported work on Sb-doped SnO2 ceramics, the ceramic resistivity shows the smallest one when the doping content Sb is 5% (in molar percentage) [9, 21, 22]. The related nominal formula of ceramic with the smallest resistivity can be defined as Sn0.95−xSb0.05O2. Here, the influence of Ga-ionic content on the electrical performance of Sn0.95Sb0.05O2 is carried out. Solid-state reaction process was used to prepared powders with nominal formula of Sn0.95−xSb0.05GaxO2 (denoted as SSGOx, x = 0, 0.01, 0.02, 0.03, 0.04 and 0.05), and the related ceramics were received by traditional sintering mothed. Raw materials of SnO2 (99.5%, Xilong Scientific Co., Ltd., China), Sb2O3 (99%, Xilong Scientific Co., Ltd., China), and Ga2O3 (99.99%, Shanghai Longjin Metal Material Co., Ltd., China) powders were weighed according to the nominal formula. The raw materials were mixed by ball milling for 1 h with deionized water as dispersion agent and then were dried in an oven at 110 °C for 12 h. The dried mixtures were calcined at 800 °C for 5 h in air. Then 1% (in weight percentage) CuO (99%, Shanghai Macklin Biochemical Co., Ltd., China) was mixed with the calcined powders to promote sintering ability. The mixed powders were fully ground and granulated with solution of polyvinyl alcohol (PVA) in deionized water. Then the granulated powders were pressed into pellets with 12 mm in diameter and around 2 mm in thickness. The green pellets were sintered at 1350 °C for 1 h to get dense ceramics. The as-sintered ceramics were polished and coated with silver on both opposite sides, followed by being heated at 600 °C for 5 min to make ohmic electrodes for measuring electrical properties.
2.2 Characterization
The phase component and lattice parameters of as-sintered ceramics were characterized by X-ray diffractometer (XRD, Rigaku D/max 2500, Japan) with Cu Kα radiation with wavelength λ of 0.154056 nm. A scanning electron microscopy (SEM, JMS-7900 F) was employed to observe the microstructure of samples, and element distribution characterization was performed by an energy dispersive X-ray spectroscopy (EDS, Oxford Ultim Max 65). The ceramics used for SEM characterization were polished, followed by thermal etching at 1200 °C for 30 min. The valence states of elements in ceramics were analyzed by X-ray photoelectron spectroscopy (XPS, K-alpha 1063, UK). The relative densities of ceramics were received with the Archimedes method.
In order to investigate the electrical properties of SSGOx ceramics, a resistance–temperature measurement system (ZWX-C, Huazhong University of Science and Technology, China) was used to measure the resistances over temperature range between 25 and 250 °C. Alternating current (AC) impedance spectroscopy was implemented using an electrochemical workstation (Gamry Reference 600, USA) in frequency range from 1 Hz to 1 MHz, and the impedance spectra were analyzed with Gamry Echem Analyst.
Aging treatment was carried out to investigate the electrical stability of ceramics. The ceramics with ohmic electrodes were put into an oven which was held at 150 °C in air. After different aging periods, the samples were taken out from the oven and placed at room temperature for at least 60 min. Then the sample resistances were measured using FLUKE 8808A. The aging-induced resistance change rate ΔR/R0 was calculated by ΔR/R0 = (R − R0)/R0, where R0 represents initial resistance of sample at 25 °C and R is sample resistance at 25 °C after being aged for some time.
3 Results and discussion
3.1 Phase and microstructure
Figure 1 illustrates SEM images of SSGO0 and SSGO0.04 ceramics. Dense ceramics are obtained, although few pores are present. The relative densities of SSGOx ceramics measured by Archimedes method are 98.8%, 96.9%, 95.6%, 95.2%, 92.7%, and 91.5% for x are 0, 0.01, 0.02, 0.03, 0.04, and 0.05, respectively. As can be seen in Fig. 1, there are two kinds of particles with different size. The particles with smaller size are distributed at the grain boundaries of the larger grains.
In order to figure out the composition distribution of different kinds of particles as shown in Fig. 1, EDS analysis was conducted for sample SSGO0.04. Figure 2 shows a SEM image and related EDS mapping of elemental distribution of thermally etched surface of SSGO4 ceramic.
The Sn element is uniformly distributed in the larger-sized particles, as shown in Fig. 2b. The Sb and Ga elements, as shown in Fig. 2c, d, are mainly distributed in the larger-sized particles, but a trace amount of them distribute in the smaller-sized particles. In the smaller-sized particles, Cu and O are the main elements (as shown in Fig. 2e, f). These indicate that the smaller-sized particles as shown in Fig. 1 are CuO-rich regions. CuO was introduced into the calcined SSGO powders as a sintering aid. Due to the low melting point of CuO (1026 °C) [12], a liquid phase was formed during the sintering process and distributed on the surface of the SSGO particles, which promoted the migration of substances and improved the ceramic density. At the same time, trace amounts of Sb and Ga that were not completely dissolved into SnO2 lattice might form solid solutions or mixtures with CuO and locate at grain boundaries. So the CuO-rich liquid-like phase was distributed at the grain boundaries [23].
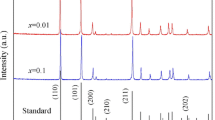
The XRD patterns of Sn0.95−xSb0.05GaxO2 (x = 0, 0.04, and 0.05) ceramics are shown in Fig. 3a. All the XRD patterns can be indexed as that of tetragonal SnO2 crystal with a space group of P42/mnm (Ref. PDF No. 99-0024). Combining with the elemental distribution in Fig. 2, it can be concluded that most of Sb ions and Ga ions had entered the lattice of SnO2, substituted at the Sn sites and formed a solid solution with SnO2. In addition, CuO-rich phase is present in ceramics, but there is no corresponding peak in the XRD patterns. It may be attributed to the detection limit of the X-ray diffractometer because the addition of CuO is only 1% in weight percentage, not enough to be detected by XRD.
Figure 3b is the partially magnified view of XRD patterns of (110) peak as shown in Fig. 3a. One can see that the (110) peak gradually shifts towards higher diffraction angle with the increase of Ga ions content, indicating that the interplanar spacing decreases. Lattice parameters and unit cell volumes of the measured ceramics are refined by Jade 6.5+ and listed in Table 1. The lattice parameters (a and c) and cell volumes decrease with the rise of Ga content. This is because that Ga3+ ions have been dissolved into SnO2 lattice and the ionic radius of Ga3+ ions (0.062 nm, in 6 coordination state) is smaller than that of Sn4+ ions (0.069 nm, in 6 coordination state) [24]. The substitution of Ga3+ ion at Sn4+-ion site induced the lattice distortion and shrinking. At the same time, substitution of Ga3+ ions would produce oxygen vacancies as shown in defect reaction equation of Eq. (1). The occurrence of oxygen vacancies leads to distortion and shrinking of related oxygen octahedrons, resulting in a reduction of lattice parameters.
Figure 3c presents the relationship between the cell volume and x, namely the concentration of Ga3+. When x increases, the unit cell volume decreases linearly. According to Kostikova’s research on Vegard law, this linear relationship further proves the presence of solid solution [6].
3.2 Electrical properties
The resistance changes with temperature of Sn0.95−xSb0.05GaxO2 (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) were measured using a resistance–temperature measurement system over the temperature range from room temperature to 250 °C. The corresponding resistivity was calculated according to formula of ρ = RS/l, where R is measured resistance, S is cross-sectional area of the circular ceramic sample, and l is the thickness of the sample perpendicular to the cross section. The plots of temperature dependence of resistivity (lnρ–1000/T) of SSGOx ceramics are shown in Fig. 4a. All SSGOx ceramics possess typical NTC characteristics, and the plot of lnρ–1000/T for each sample has an approximate linear relationship. The relationship between lnρ and 1000/T obeys the Arrhenius law as shown in Eq. (2).
where ρ is resistivity at absolute temperature T, A is a constant relevant to material characteristic, Ea is activation energy of conduction, k is the Boltzmann constant (which is 1.38 × 10−23 J/K), and B is NTC material constant reflecting the temperature sensitivity of a NTC thermistor. For NTC thermistors, B value can be calculated according to Eq. (3).
where ρ25 and ρ85 are resistivity at 25 °C (298 K) and 85 °C (358 K), respectively. So the B value is often denoted as B25/85.
Figure 4b shows plots of ρ25 and B25/85 as a function of Ga concentration in SSGOx ceramics. The ρ25 of SSGOx are 3.2 Ω·cm, 24 Ω·cm, 3.15 × 102 Ω·cm, 4 × 103 Ω·cm, 3.5 × 105 Ω·cm, and 5.3 × 105 Ω·cm for x are 0, 0.01, 0.02, 0.03, 0.04, and 0.05, respectively. The related B25/85 are 140 K, 1161 K, 2214 K, 3735 K, 5384 K, and 5560 K, individually. With the increase of Ga content, both ρ25 and B25/85 increase.
Generally, the substitution of heterovalent ions into a crystal may induce the occurrence of point defects and charge carriers. When higher valent ion donors are introduced, positively charged point defects and electrons will be generated, and the produced electrons may be excited to the conduction band of the semiconductor to form electron carriers. Negatively charged point defects and holes are generated when a crystal is doped with lower-valent ion acceptor, and holes may be excited from the acceptor energy level to the valence band, thus, playing the role of conduction. In the experiment, Sb2O3 was selected as the starting material, and Sb2O3 can be easily oxidized to Sb2O5 during calcination and sintering [25]. In SSGO system, both donor and acceptor doping are introduced into SnO2 crystal lattice, in which Sb5+ acts as donor, Ga3+ and Sb3+ ions act as acceptors. For the doping of Sb5+ ions into SnO2 lattice, defect reaction may take place as shown in Eq. (4).
Here, Sb2O5 acts as a donor and produces weakly bound electron carriers, thereby enhancing the conductivity of the ceramics. These electrons are located in the donor energy level, which is closer to the conduction band than the valence band, so the electrons can be excited to the conduction band more easily. The activation energy required for electron conduction is reduced, and the material constant B25/85 decreases.
When Ga3+ ion dops into SnO2, Ga3+ replaces Sn4+ ions to generate holes, the related defect reaction is shown in Eq. (5).
The holes react with electrons generated by Sb5+ doping to form electron–hole pair, or the electrons in conduction band (or donor level) transition to valence band (or acceptor level) to recombine with holes. Those processes reduce the concentration of electron charge carriers shown in Eq. (4). Therefore, the resistivity increases with the rise of Ga concentration.
As shown in Eq. (1), the substitution of Ga3+ ions at Sn4+ sites induced the formation of oxygen vacancies, and resulted in lattice distortion and increase of location lattice potential. So the charge carriers need more energy to transfer the lattice-distorted regions, indicating that the activation energy of conduction inside grains (Eg) is bigger in Ga-doped ceramics than in the undoped one. At the same time, for the different valences between Ga3+ and Sn4+ ions, the increase of Ga-ion content might exceed the solid solution limit of Ga2O3 in SnO2, and results in the occurrence of impurity locating at grain boundaries. The impurity should be composed of various elements such as Sb-, Ga-, and Cu-ions, and is always poor conductor to the charge carriers (electron or hole). As a result, the potential barrier of grain boundary increases with the increase of Ga-ion content. So the charge carriers transfer over the grain boundaries must overcome higher potential, resulting in higher activation energy of conduction at grain boundaries (Egb). The total activation energy of conduction (Ea) is composed of Eg and Egb, e.g., Ea = Eg + Egb. Therefore, the higher concentration of Ga ions is, the higher Ea is. According to Eq. (2), the material constant B is proportional to Ea, so the B25/85 value increases with increasing Ga concentration.
Table 2 shows comparison of electrical properties of Sn0.95−xSb0.05GaxO2 and some published NTC materials. Both ρ25 and B25/85 of Sn0.95−xSb0.05GaxO2 can be adjusted in a wider range only by changing the concentration of Ga3+-ions and have obviously larger adjustable regions than those in perovskite or spinel compounds. These indicate that the system of Ga-doped Sn0.95–Sb0.05O2 (Sn0.95−xSb0.05GaxO2) NTC thermistors have potential wide application prospect to actual requirements.
3.3 AC impedance analysis
To further analyze the reason for the Ga content on the resistances of ceramics, AC impedance test was performed on SSGOx (x = 0.02, 0.03, 0.04, 0.05) at room temperature. The impedance Z* can be described by Z* = Z′ + jZ″, where Z′ is real part of impedance, and Z″ is imaginary part of impedance. The impedance in Nyquist plots are shown in Fig. 5a, b. The impedance in Nyquist plot of ideal polycrystal consists of two semicircles, corresponding to grain and grain boundary, respectively. Only one depressed semicircle can be observed in Fig. 5, because the arcs of the grain and grain boundary overlap. In order to reveal the characteristic of the complex impedance spectra, an equivalent circuit as the inset in Fig. 5a was selected to fit the spectra. R1 and R2 represent resistances from grain effect and grain boundary effect, respectively, C1 and C2 are respective capacitance of grain effect and grain boundary effect, and CPE1 and CPE2 are constant phase elements. It can be seen that the fitting results are in good agreement with the measured data. The Ga concentration dependence of fitted resistances of grain effect and grain boundary effect is shown in Fig. 5c. With the increase of Ga content, both the resistances of grain effect and grain boundary effect simultaneously increase. The increase of grain resistance results from more holes generated with the increase of Ga content, as shown in the defect reaction in Eq. (5). The recombination of electrons and generated holes reduces the amount of carriers (electrons) in the grain, thereby increasing the grain resistance.
Analysis of complex impedance spectra of SSGOx ceramics, measured at room temperature, a Nyquist plots of SSGOx (x = 0.02, 0.03), the inset shows the equivalent fitting circuit, b Nyquist plots of SSGOx (x = 0.04, 0.05), and c Ga concentration dependence of resistances of grain and grain boundary effect
The increase of grain boundary resistance with Ga addition should be attributed to the variation of grain boundary barrier. When charge carriers cross grain boundary, they should overcome grain boundary barrier (Egb). According to the Boltzmann statistical distribution law, the probability for a charge carrier to reach Egb is proportional to exp(− Egb/kT), meaning that the chance to overcome the barrier is exponentially relevant to Egb. For the limited solid solubility of Sb/Ga in SnO2, impurity must form when the content of dopants (Sb and Ga ions) is high enough. With the increase of Ga-ion content, more impurity might occur and locate at grain boundaries. The impurity should be composed of various elements such as Sb, Ga, and Cu ions and is always poor conductor to the charge carriers (electron or hole). As a result, the potential barrier of grain boundary increases. So it is more difficult for carriers to transition at grain boundary, leading to higher grain boundary resistance and Egb.
In order to figure out the conduction mechanism of SSGOx ceramics, analysis of SSGO0.04 sample’s impedance spectra recorded at different temperatures was performed. Figure 6a, b is the Nyquist plots of the SSGO0.04 sample at different temperatures. The measured impedance spectra were fitted using the inset in Fig. 5a as the equivalent circuit. The fitting results are in good agreement with the measured impedance spectra. As shown in Fig. 6c, the fitted grain, grain boundary, and total resistances decrease with increasing temperature, consistent with the analysis of Fig. 4a, confirming the NTC characteristic of the ceramics. The corresponding activation energy was acquired according to Eq. (2), which has been marked in Fig. 6c. The activation energies of grain effect and grain boundary effect are 0.41 eV and 0.32 eV, respectively. This indicates that the electrical behavior of the ceramics is affected by both grain effect and grain boundary effect.
Analysis of impedance spectra of Sn0.91Sb0.05Ga0.04O2 ceramic measured at various temperatures, a and b Nyquist plots at different temperatures, c temperature dependence of Rg, Rgb, and Rg + Rgb, d plots for frequency dependence of the imaginary impedance part Z″, e angular frequency ω dependence of complex conductivity σ′(ω), and f log[σ′(ω) − σ′(0)]– logω plots
Figure 6d is plots of frequency dependence of the normalized imaginary part of impedance (Z″/Z″max). The relationship between Z″ and frequency follows
where ω is angular frequency, τ is time constant, and R is resistance. When Z″ reaches a maximum value, the corresponding
where ωc is characteristic angular frequency and fc is characteristic frequency. It can be seen from Fig. 6d that with the increase of temperature, fc gradually increases and the time constant decreases, indicating the existence of thermally activated conduction.
The admittance (σ) is inversely proportional to impedance (Z) with the relationship of σ = 1/Z. The real part of σ obeys the Jonscher's power law [14]:
where σ'(ω) represents real part of conductivity, ω is angular frequency, and σ'(0) is the DC conductivity which can be extracted from the value of σ'(ω) when ω approaches to zero, D is a constant related to temperature. n is power exponent that depends on temperature and material characteristic ranging from 0 to 1. The relationship of logσ'(ω) and logω is illustrated in Fig. 6e. The σ′(ω) is frequency independent in lower-frequency region, but increases with frequency in higher-frequency region after a critical point. The variation of σ′(ω) with frequency indicates that in addition to band conduction, there is another frequency-dependent conduction mechanism. Figure 6f shows the relationship between log[σ′(ω) − σ′(0)] and logω. n can be obtained by calculating the slope of the fitting lines. The values of n are 0.5778, 0.5654, 0.5508, 0.5534, 0.5870, 0.6818, 0.7625, and 0.9712 at temperatures of 30, 50, 75, 100, 125, 150, 175, and 200 °C, respectively. The n values are less than 1 at all temperatures, indicating the presence of hopping conduction assisted by small polaron hopping mechanism at higher frequency [28]. In Sn0.95−xSb0.05GaxO2 system, Sb ions possess both + 3 and + 5 valences. The virtual Sb4+ is proposed to explain the hopping behavior between Sb3+ and Sb5+ [29]. The mechanism of hopping conduction can be expressed by Eq. (7).
As discussed above, band conduction and hopping conduction simultaneously act on the conduction behavior of SSGOx ceramics.
3.4 Ageing properties
The variation of resistance change rates (ΔR/R0) of SSGOx (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) ceramics during aging process are presented in Fig. 7a. It indicates that the resistances of all samples decreased slightly with increasing of aging time. And the absolute values of ΔR/R0 increased as the concentration of Ga in SSGOx increases. After 500 h of aging treatment at 150 °C, the ΔR/R0 values of SSGOx are − 0.40% (x = 0), -0.69% (x = 0.01), − 0.76% (x = 0.02), − 0.88% (x = 0.03), − 1.02% (x = 0.04), and − 1.40% (x = 0.05).
To investigate the variation of grain effect and grain boundary effect induced by aging, AC impedance analysis for the same SSGO0.04 sample was conducted before and after aging. The results are shown in Fig. 7b. The equivalent circuit as the inset one in Fig. 5a was selected. The fitted grain resistances before and after aging are 10,326 and 10,189 Ω, respectively. The fitted grain boundary resistances before and after aging are 13,005 and 12,903 Ω, respectively. Both resistances of grain effect and grain boundary effect decreased after aging. The total resistance change rate is − 1.02%. The resistance change rates of grain and the grain boundary are − 1.38% and − 0.78%, indicating that the change of ceramic resistance results from both the grain and grain boundary, and the grain plays the main role.
Figure 8 shows the comparative XPS analysis of SSGO0 and SSGO0.04 ceramics before and after aging. Figure 8a and b are narrow spectra of Sn element in SSGO0 and SSGO0.04 ceramics, respectively, before and after aging. Sn element has only a + 4 valence state in all samples, and there is no change in valence state before and after aging.
In Fig. 8c (for SSGO0) and 8d (for SSGO0.04), each Sb 3d3/2 XPS spectrum can be divided into two peaks. The peak locates between 537.7 eV and 538.8 eV corresponds to Sb3+ and the one between 540.4 eV and 540.6 eV corresponds to Sb5+. Compared the areas of fitted peaks, the content ratios of Sb5+ to Sb3+ ions (denoted as [Sb5+]/[Sb3+]) changed from 0.31 to 0.29 for SSGO0 ceramic and from 0.58 to 0.38 for SSGO0.04 ceramic. The [Sb5+]/[Sb3+] in both ceramics decreased after aging.
Figure 8e and f is narrow spectra of Cu element before and after aging in SSGO0 and SSGO0.04 samples, respectively. The peaks located at 932.4 eV and 952.2 eV correspond to 2p1/2 and 2p3/2 peaks of Cu+, respectively. The peaks at 933.7 eV and 953.9 eV correspond to 2p1/2 and 2p3/2 peaks of Cu2+, respectively. And the peaks at 940.4 eV and 943.3 eV are satellite peaks of Cu2+. The content ratio of Cu2+ and Cu+ ions can be obtained by calculating the area ratio of related XPS peaks. For SSGO0 sample, the content ratio of Cu2+ and Cu+ ions (denoted as [Cu2+]/[Cu+]) decreased from 0.99 to 0.83 after aging, while the one of [Cu2+]/[Cu+] in the SSGO0.04 sample decreased from 0.61 to 0.41.
Combined with the change of content ratios of [Sb5+]/[Sb3+] and [Cu2+]/[Cu+] before and after aging, one can get that the valence states of Sb ions and Cu ions had been reduced during the aging process. The decrease in resistance of the samples after aging should be due to the increase of electrons in the ceramics. The reason for the increase of electrons may be attributed to the adsorption and reaction of the moisture (water) during aging process. When the aging test was performed on the samples in the air, the moisture would be adsorbed on the surface of the ceramic and then react with the holes in the acceptor level generated by Ga3+ doping [13], as shown in Eqs. (8) and (9). Since holes were consumed by adsorbed water, more electrons would be generated in order to maintain electrical neutrality. Some of the electrons generated during aging reacted with Sb5+ to form Sb3+, the others acted as carriers for conduction together with the original electrons. Consequently, not only the ratio of Sb5+/Sb3+ reduced, but also the resistance of the ceramic decreased after aging due to the increase of carrier (electrons) concentration.
Similar to Sb ions, the change in the valence state of Cu ions is also due to the presence of more electrons in the ceramic, except that the electrons are mainly from the grain boundary. The water adsorbed on the ceramic surface reacted with the holes at the grain boundary, as shown in Eqs. (8) and (9). In order to maintain electrical neutrality, more electrons were generated, some of which reacted with Cu2+ to form Cu+, the others acted as carriers. Therefore, the content ratio of Cu2+/Cu+ reduced, and the grain boundary resistance also decreased due to the increase of carrier concentration. In addition, the ratio changes of Sb5+/Sb3+ and Cu2+/Cu+ in SSGO0 are less than those in SSGO0.04, respectively, which is consistent with the resistance change rate of both samples.
Although the resistance of SSGO ceramic samples decreases after aging, the resistance change rate is less than − 1.4% for all samples, indicating that the samples have stable electrical properties.
4 Conclusion
Sn0.95−xSb0.05GaxO2 (0 ≤ x ≤ 0.05) ceramics were synthesized with solid-state processes. All of the compositions exhibit typical NTC characteristic. Changing the concentration of Ga ions can effectively adjust room-temperature resistivity from 3.24 Ω·cm to 0.54 MΩ·cm and temperature sensitivity (material constant B value) from 140 to 5384 K of the ceramics. The prepared NTC ceramics possess stable electric properties, the resistance change ratio ΔR/R0 is less than 1.4% even after being aged at 150 °C for 500 h. These make that the Sn0.95−xSb0.05GaxO2 NTC thermistors have potential application. Band conduction and hopping conduction are responsible for electrical properties of the ceramics. The adsorption and reaction of water in the air are the reason for the resistance change after aging.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
L. Chen, W. Kong, J. Yao, B. Gao, Q. Zhang, H. Bu, A. Chang, C. Jiang, Effect of sintering temperature on microstructure and electrical properties of Mn1.2Co1.5Ni0.3O4 ceramic materials using nanoparticles by reverse microemulsion method. J. Mater. Sci. Mater. Electron. 27, 1713–1718 (2015)
C. Ma, H. Gao, TEM and electrical properties characterizations of Co0.98Mn2.02O4 NTC ceramic. J. Alloys Compd. 749, 853–858 (2018)
R. Jadhav, D. Kulkarni, V. Puri, Structural and electrical properties of fritless Ni(1–x)CuxMn2O4 (0 ≤ x ≤ 1) thick film NTC ceramic. J. Mater. Sci. Mater. Electron. 21, 503–508 (2009)
J. Wang, J. Zhang, Structural and electrical properties of NiMgxMn2−xO4 NTC thermistors prepared by using sol–gel derived powders. Mater. Sci. Eng. B 176, 616–619 (2011)
P. Luo, B. Zhang, Q. Zhao, D. He, A. Chang, Characterization and electrical conductivity of La1−xSrxCrO3 NTC ceramics. J. Mater. Sci. Mater. Electron. 28, 9265–9271 (2017)
F. Guan, Z. Dang, X. Chen, S. Huang, J. Wang, X. Cheng, Y. Wu, Novel electrical properties of Mn-doped LaCrO3 ceramics as NTC thermistors. J. Alloys Compd. 871, 159269 (2021)
Y. Luo, X. Liu, G. Chen, Electrical properties of BaTiO3-based NTC thermistors doped by BaBiO3 and CeO2. J. Alloys Compd. 429, 335–337 (2007)
J. Qu, S. Li, F. Liu, X. Liu, Z. Chen, C. Yuan, X. Liu, Y. Zhao, D. Zhou, Effect of phase structures and substrate temperatures on NTC characteristics of Cu-modified Ba–Bi–O-based perovskite-type thermistor thin films. Mater. Sci. Semicond. Process. 121, 105375 (2021)
W. Yan, H. Zhang, X. Wang, C. You, Z. Li, Characterization of electrical conductivity and temperature sensitivity of Cr/Sb-modified SnO2 ceramics. J. Mater. Sci. Mater. Electron. 31, 4040–4049 (2020)
Z. Yang, H. Zhang, Z. He, B. Li, Z. Li, Influence of B3+- and Na+-ions on electrical property and temperature sensitivity of NiO-based ceramics. J. Mater. Sci. Mater. Electron. 30, 3088–3097 (2019)
C. You, Z. Li, S. Zhang, G. Jiang, H. Zhang, Electrical properties of Sr-modified CuO ceramics. J. Mater. Sci. Mater. Electron. 32, 15907–15916 (2021)
P. Li, H. Zhang, C. Gao, G. Jiang, Z. Li, Electrical property of Al/La/Cu modified ZnO-based negative temperature coefficient (NTC) ceramics with high ageing stability. J. Mater. Sci. Mater. Electron. 30, 19598–19608 (2019)
B. Li, Z. Li, D. Peng, L. Huang, S. Zhang, H. Zhang, Sb-doped ZnO ceramics: NTC thermistors with high temperature sensitivity and electrical stability. J. Mater. Sci. Mater. Electron. 32, 24296–24307 (2021)
X. Sun, S. Leng, H. Zhang, Z. He, Z. Li, Electrical properties and temperature sensitivity of Li/Mg modified Ni0.7Zn0.3O based ceramics. J. Alloys Compd. 763, 975–982 (2018)
W.A. Groen, C. Metzmacher, V. Zaspalis, P. Huppertz, S. Schuurman, Aging of NTC ceramics in the system Mn–Ni–Fe–O. J. Eur. Ceram. Soc. 21, 1793–1796 (2001)
H. Li, I.P.L. Thayil, X. Ma, X. Sang, H. Zhang, A. Chang, Electrical properties and aging behavior of Na-doped Mn1.95Co0.21Ni0.84O4 NTC ceramics. Ceram. Int. 46, 24365–24370 (2020)
W. Ren, N. Zhu, L. Li, H. Feng, Y. Wang, Y. Yang, Z. Yang, S. Shang, Improvement of ageing issue in Zn0.4Fe2.1Co2Mn1.5O8 thermistor films. J. Eur. Ceram. Soc. 39, 4189–4193 (2019)
C. Gao, Z. Li, L. Yang, D. Peng, H. Zhang, Investigation of electrical and aging properties of Bi-modified (Zn0.4Ni0.6)1−xNaxO ceramic thermistors. J. Eur. Ceram. Soc. 41, 4160–4166 (2021)
M.M. Vakiv, O.I. Shpotyuk, V.O. Balitska, B. Butkiewicz, L.I. Shpotyuk, Ageing behavior of electrical resistance in manganite NTC ceramics. J. Eur. Ceram. Soc. 24, 1243–1246 (2004)
D. Fang, C. Zheng, C. Chen, A.J.A. Winnubst, Aging of nickel manganite NTC ceramics. J. Electroceram. 22, 421–427 (2008)
G. Jiang, Z. Li, C. You, W. Hao, Z. Ma, H. Zhang, Temperature sensitivity and electrical stability of Sb/Mn co-doped SnO2 ceramics. J. Mater. Sci. Mater. Electron. 32, 16945–16955 (2021)
J. Zhang, H. Zhang, B. Yang, Y. Zhang, Z. Li, Temperature sensitivity of Fe-substituted SnO2-based ceramics as negative temperature coefficient thermistors. J. Mater. Sci. Mater. Electron. 27, 4935–4942 (2016)
T.S. Zhang, L.B. Kong, X.C. Song, Z.H. Du, W.Q. Xu, S. Li, Densification behaviour and sintering mechanisms of Cu- or Co-doped SnO2: a comparative study. Acta Mater. 62, 81–88 (2014)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 32, 751–767 (1976)
S. Gong, Z. Chen, F. Tang, P. Pi, X. Wen, Preparation and characterization of antimony doped tin oxide nanoparticles. J. Chin. Ceram. Soc. 37, 648–652 (2009)
Z. Wang, Z. Li, Y. Zhang, R. Zhang, P. Qin, C. Chen, L. Winnubst, Preparation and electrical properties of Ni0.6Mn2.4−xSnxO4 NTC ceramics. Ceram. Int. 40, 4875–4878 (2014)
B.Y. Price, G. Hardal, Preparation and characterization of Ni–Co–Zn–Mn–O negative temperature coefficient thermistors with B2O3 addition. J. Mater. Sci. Mater. Electron. 30, 17432–17439 (2019)
R. Kaur, V. Sharma, M. Kumar, M. Singh, A. Singh, Conductivity relaxation in Pb0.9Sm0.10Zr0.405Ti0.495Fe0.10O3 solid solution. J. Alloys Compd. 735, 1472–1479 (2018)
M.A.L. Nobre, S. Lanfredi, Dielectric spectroscopy on Bi3Zn2Sb3O14 ceramic: an approach based on the complex impedance. J. Phys. Chem. Solids 64, 2457–2464 (2003)
Funding
This work is supported by the research funds from the National Natural Science Foundation of China (No. 51767021), Fundamental Research Funds for the Central Universities of Central South University (No. 2022ZZTS0532), Foundation of the Department of Science and Technology of Guizhou Province (No. CG[2021]110), and Development Funds of Hunan Wedid Materials Technology Co., Ltd., China (No. 738010241).
Author information
Authors and Affiliations
Contributions
ZL, HZ, and SZ contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SZ, SL, YW, and HW. The first draft of the manuscript was written by SZ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Zhang, H., Leng, S. et al. Electrical properties and aging characteristic of Sb/Ga co-doped single-cation oxide SnO2. J Mater Sci: Mater Electron 33, 23821–23833 (2022). https://doi.org/10.1007/s10854-022-09140-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09140-6