Abstract
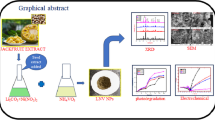
LiZnVO4 nanoparticles (NPs) were synthesized by a simple combustion method using Hibiscus Rosa Sinensis leaves as a novel fuel and the prepared NPs were characterized using XRD, FT-IR, morphological analysis by SEM and TEM studies, elemental analysis by EDAX and ICP-MS. XRD data of the synthesized NPs were well-matched with the JCPDS number 038–1332 and it confirms the distorted phenacite structure of LiZnVO4. FT-IR strengthens the bonding of M-M and M–O in LiZnVO4. Optical characteristics of LiZnVO4 NPs were studied by photoluminescence (PL) spectroscopy. Vanadate group of the prepared NPs originates a green emission. Examination of latent finger prints (LFP’s) showed a crystal clear patterns under near UV region (365 nm) as they are the prominent techniques in crime investigations. LiZnVO4 NPs exhibit a strong antioxidant property and act as a good sensor to detect sodium nitrite analyte at extremely low concentrations with limit of detection of 27.5 nM. Three-electrode system supercapacitor device has been fabricated and examined the performances. Specific capacitance of LiZnVO4 NPs based supercapacitor was found to be 88.7 F/g at a current density of 0.1 mA. Power density (Pd) and energy density (Ed) were found to be 0.51 W/g and 12.3 Wh/Kg, respectively. This device exhibits a stable CV curves up to the scan rate of 10 V/s. Furthermore, LiZnVO4 NPs acts as an excellent material towards the humidity sensing with a sensitivity factor, sensitivity and limit of detection of 28.0, 0.3 MΩ/%RH and 5%RH, respectively. All the above studies show the diverse potentiality of LiZnVO4 NPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
At present, nanotechnology is one of the leading fields in analytical technique of chemical science. Metal oxide nanoparticles found diverse applications for the fabrication of analytical tools. In that direction, materials scientists were observed that mixed metal oxides shows enhanced performances in comparison with its metal oxides. Recent literature survey and also of research interest allows to select LiZnVO4 as one of the prominent materials to explore diverse applications including biological, chemical and medicinal fields. But in chemical, development of antioxidants is essential in annihilating several diseases as they slow down the activity of hazardous reactive groups such as reactive oxygen species (ROS) which includes hydroxyl radicals (·OH), superoxide and peroxide radicals (O2·– and O2·2–), singlet oxygen (1O2) and peroxyl radicals [1]. Hence, a substantial research is focused on the conversion of nanomaterials into useful antioxidants.
Electrochemical sensing can be used as the technique for detecting and measuring the analyte at different concentration level. Depending on the unique features of nanomaterials, they have been extensively used in many applications. Also, they play characteristic roles in several electrochemical sensing methods such as promotion of electron transfer, catalysis, labeling of biomolecules, as a reactant and in the immobilization process of several biomolecules [2]. Sodium nitrite is weakly toxic. In animal tissues, nitric oxide, a neurotransmitter, undergoes metabolism to produce nitrite. Sodium nitrite leads methemoglobinemia in omnivorous animal like swine and lowers the quantity of oxygen liberated from haemoglobin, in this way the animal lacks its strength and then die [3]. It has the ability to bring about tumors and grow up the malignancy. Therefore, quantification of nitrite at low concentration level is essential. Various methods are available to probe this analyte. However, electrochemical detection is more achievable when contrasted to other methods [4]. Nanomaterials modified sensors are effective in detecting sodium nitrite at very low concentration levels by employing electrochemical voltammetric technique.
Energy is truly necessary these days as energy issues are a big challenge in the twenty-first century. Supercapacitors are considered as excellent devices for the storage of energy due to their high power density, high load currents, fast charging, long cycling life, operational safety, etc., hence, the development of them is very essential. Humidity sensors found most useful applications such as in Pharmaceuticals, agriculture, food processing, etc., which estimates the presence of moisture content and also the material’s resistance there by its sensitivity can be determined. The higher the humidity sensing activity of the material, the higher will be the capacitance of it. In this report, efforts have been made in developing the LiZnVO4 based supercapacitors and in determining the specific capacitance by a simple procedure using cyclic voltammetry and galvanostatic charge and discharge technique.
LiZnVO4, a trimetallic oxide, a vanadate based material, found applications in energy storage devices such as Li-ion battery [5,6,7] and optical properties [8] due to its arrangement of atoms in a crystal lattice and advanced redox chemistry of vanadate group. Zeng et al. have synthesized it from hydrothermal method [5] for Li-battery applications. Yunyan et al. have synthesized it from solid state reaction at high temperature of 750 °C [6] for electrochemical measurements. In this report, we have emphasized the synthesis by simple, non chemical, green fuel assisted, solution combustion route using Hibiscus rosa sinensis leaves as fuel. This method is exceptionally easy to produce the compound in mass for huge scope modern applications as other methods demand tedious procedures, time consuming and thorough experimental conditions such as high sintering and annealing temperatures and the possibilities of obtaining impurities are high in solid state synthesis. We are the first to report the antioxidant activity, Latent fingerprint development for crime investigations using a technique of plain LiZnVO4 NPs based powder dusting technology, supercapacitor behavior and sensing activity of LiZnVO4 NPs towards the detection of nitrite at very low concentration and Humidity sensing of pure LiZnVO4 NPs.
2 Experimental details
2.1 Preparation of LiZnVO4 NPs
For the synthesis of LiZnVO4 NPs by solution combustion route, precursors such as lithium nitrate, zinc nitrate and ammonium metavanadate are required (Merck India Ltd). Finely powdered hibiscus leaves were used as a fuel. Stoichiometric amount of the above precursors in 1:1:1 ratio along with the finely powdered hibiscus leaves of 600 mg were taken in the silica crucible having 3 mL of distilled water. The contents are stirred magnetically and introduced into the preheated, 500 °C temperature asserted muffle furnace. Combustion process was concluded in 5 min with the formation of yellowish white LiZnVO4 NPs as a product.
2.2 Characterization
Crystal structure of the LiZnVO4 NPs were analyzed by recording the XRD data of the sample using Rigaku-Smart Lab X-ray diffractometer with CuKα (1.541 Å) radiation. FT-IR spectrum of the prepared sample was obtained with the aid of Bruker Alpha-p spectrometer from KBr pellet method. The surface morphology was analyzed using (Hitachi – 3000) Scanning electron microscope. TEM images were collected using FEI tecnai model. Elemental and trace elemental analysis were made by using EDAX and ICP-MS. Photoluminescence of LiZnVO4 NPs was investigated by Cary eclipse fluorescence spectrophotometer (Agilent technologies). Developed LFP’s on different surfaces were visualized through UV cabinet at 365 nm and the corresponding pictures were captured by Samsung Galaxy J7 rear camera with 13-mega pixels.
2.3 Antioxidant activity
Antioxidant activity of the prepared LiZnVO4 NPs was studied by following the modified process of Brand-Williams [9] using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. DPPH is a stable free radical, which is violet in color corresponding to its oxidized form. It exhibits a broad peak due to the maximum absorption in the visible region (520 nm). As soon as an antioxidant entity gives an electron to the DPPH radical and its action was hindered. Consequently, adjustments in absorbance take place at the same wavelength. 19.7 mg of DPPH was liquefied in 50 mL methanol to acquire 0.14 mM concentration of DPPH. 50% methanol was prepared by diluting methanol with de-ionized water (1:1). Different concentrations of the test sample, such as, 20, 40, 60 and 80 μg were set up by blending relative volumes of sonicated LiZnVO4. To this, 50% methanol was added such that it should make 1 mL when 140 μL of DPPH solution was added. The blend was incubated at 35 °C for about 30 min. A blank solution was prepared without adding the test sample, which is considered to be a control sample. Using a spectrophotometer, the absorbance of the samples was recorded at 520 nm, with reference to the blank solution. The difference between the absorbance of the blank solution and the test sample gives the actual absorbance, which is useful in estimating the percentage inhibition value of an antioxidant. By plotting the percentage inhibition against the concentration of the NPs, Inhibition concentration-50 (IC50) can be determined.
2.4 Electrode modification and their electrochemical examination
2.4.1 Experimental
Complete electrochemical analysis was performed using Inc (Chi6111e) CH Instruments and an electrochemical cell of capacity 10 mL provided with three-electrode system, in which working electrode (glassy carbon electrode), auxiliary electrode (a platinum wire) and reference electrode (Ag/AgCl) were incorporated. pH of the solution was examined by employing a pH meter. 1 M NaOH aqueous solution and the potential range from − 1.2 to 1.2 V were set to obtain the CV curves. Specific capacitance (Cs) and the Energy density (Ed) of the prepared electrodes were calculated from the CV data using the Eqs. (1) and (2) [10]
where IdV represents the area of the CV, m is the mass of the active material deposited on the electrode, ΔV is the potential window (2.4 V) and ν is the selected scan rate (in mVs−1).
2.4.2 Surface modification
Before modification, the glassy carbon electrode (GCE) was rinsed with DI water and polished manually with various grades of alumina particles to get a mirror finish. Then it was subjected to sonication with deionized water and ethanol for about 2 min. The dispersed solution of the LiZnVO4 NPs was made by sonicating the solution of 5 mg of it in 1 mL of DI water. Then the corresponding solution was coated on the surface of the GCE by drop casting method and kept aside to air dry for 4 h.
3 Results and discussion
3.1 XRD studies
Premier characterization of LiZnVO4 NPs was done using XRD studies as outlined in Fig. 1. The peaks of diffraction patterns are indexed to the rhombohedral crystal system with distorted phenacite structure, as the data obtained are in accordance with the JCPDS card number 038–1332. From the Debye–Scherrer Eq. (3), the average crystallite size of LiZnVO4 NPs was estimated to be 67 nm.
where, D is the average grain size or crystallite size, λ signifies the wavelength of the X-ray, K is the crystallite shape factor (0.94), β is the full width at half maximum and θ is the Bragg’s diffracting angle [11].
3.2 FT-IR studies
Synthesized LiZnVO4 NPs were subjected to FT-IR analysis and the spectrum was recorded between 350 and 4000 cm−1 (Fig. 2). Different functional groups and the existence of metal–oxygen bonds in the sample were investigated from the FT-IR analysis. A huge vibration band ranging from 400 to 700 cm−1 is referred to the stretching of Zn–O bond [12]. The band at 480 cm−1 represents the vibrational stretching frequency of Li-VO4 group [13]. Peak at 813 cm−1, corresponding to V–O-V vibration of vanadate group [14]. Peaks at 1637 and 2920 cm−1 corresponds to C = C and C-H stretching, respectively. A peak corresponding to 3416 cm−1 specifies the O–H stretching and a less intense peak at 1637 cm−1 demonstrates the bending vibrations of O–H bonds, indicating the presence of moisture in the sample [15].
3.3 SEM studies
Figure 3. depicts the SEM image and EDAX of LiZnVO4 NPs. SEM image distinctly exemplifies the cluster of particles formed from the agglomeration of NPs. They are more or less spherical in shape. The composition of LiZnVO4 includes Zn, V and O as specified by EDAX.
3.4 TEM studies
Figure 4. shows the (a-c) TEM images, (d) HR-TEM and (e) SAED pattern of LiZnVO4 NPs. The sharp edge structure of the phenacite LiZnVO4 crystal is evident in the TEM images. The plane obtained from the d-spacing value of the HR-TEM image is well-matched with the (110) plane of the XRD pattern. Bright spots in the SAED pattern indicate the high crystalline nature of the material and the presence of XRD planes (110), (220), and (520) is confirmed by the SEAD pattern.
3.5 ICP-MS studies
To detect the metals present in the liquid sample of LiZnVO4 NPs, a chemical characterization was carried out with the aid of inductive coupled plasma mass spectrometry studies (ICP-MS). Liquid sample was prepared at extremely low concentrations by digesting solid LiZnVO4 NPs in con. HNO3 and water for 20 min at 80 °C with dilution factor of 1000. Blank solution of ‘0’ ppb and concentration of 100 ppb solution was prepared and used as standard solution. Certified concentrations of Li, Zn and V with concentration and uncertainty of 100.0 ± 0.5 mg/L were used as reference. The following table and Fig. 5. Illustrate the concentration of elements present in the liquid sample.
4 Photoluminescence studies
Semiconductor’s charge separation and the electronic structure of the material can be probed by PL studies. The excitation and emission spectrum of LiZnVO4 NPs show a band at 337 nm and 533 nm, respectively, as depicted in Fig. 6a and b. A peak was observed at 324 nm in the excitation spectrum. CIE (Commission International D I’Eclairage) diagram was plotted (Fig. 6c), the color of light emission was found to be light green, and it is indicated by an asterisk mark on the CIE diagram. The chromaticity coordinates were estimated to be X = 0.33101 and Y = 0.45136 which is suited for green emission and these factors proves that the prepared LiZnVO4 NPs can be successfully used for the Light Emitting Diode applications (LEDs) [16,17,18].
4.1 Latent fingerprint applications
We all know that human fingerprints are extremely unique. It differentiates from person to person and hence this feature is most importantly used in forensic applications. But LFP’s are not visible to the naked eye and require additional processing techniques. LFP’s may be developed from several methods such as powder dusting, ninhydrin dipping, iodine fuming and silver nitrate soaking [19, 20]. All these methods have their own disadvantages like low resolution, cost effect and background noise. In the present study, we have utilized the method of powder dusting which is easier and quick method. Scheme 1 explains the procedure for the development of LFP’s. LFP’s were created on rigid surfaces such as a beaker, a CD, and a knife. The represented fingerprints contain eye, loops, whorls, and arches. Additionally, the clear ridge structures of the Fingerprints can be clearly detected without any obstruction. As a result, the prepared LiZnVO4 NPs can be regarded as the best material for LFP’s applications (Fig. 7).
5 Antioxidant studies
DPPH is generally used to assess the capacity of compounds to function as free radical scavengers or hydrogen radical donors and to estimate the antioxidant activity [21]. The principle behind the molecule showing antioxidant property can be depicted as; in the presence of antioxidants, DPPH diverges from violet to yellow color analogous to its reduced form [22]. This is because of the oxidation of prepared NPs and reduction of the DPPH radical by the movement of either electrons or hydrogen radicals from the sample, neutralize the DPPH and then DPPH turns yellow color, as depicted in the scheme 2.
By observing the concentration of DPPH and furthermore, by estimating the decrement in the DPPH absorbance around 520 nm, antioxidant activity of the sample was assessed [23].
The percentage (%) of inhibition of the DPPH can be determined using Eq. (4)
where Ai is the absorbance of the control and Ao is the absorbance of the sample solution [24].
From the Fig. 8, IC50 value was found to be 37.32 μg/mL which is very low and accordingly the synthesized LiZnVO4 NPs can be utilized as an acceptable antioxidant agent [25].
6 Electrochemical functioning of LiZnVO4 NPs: sensing of sodium nitrite
In order to examine the electrochemical performance of the prepared LiZnVO4 NPs, cyclic voltammetric technique has been adopted using sodium nitrite as a model system. CV’s of nitrite at bare GCE, at GCE with 5 mM concentration of nitrite and LiZnVO4 coated GCE with 5 mM of nitrite solution were recorded using phosphate buffer of pH 12.0 with a scan rate of 50 mV/s, shown in Fig. 9. A kind of simple line was observed with bare GCE, representing an action of electron transfer has not yet started because of the absence of the analyte (Curve c in Fig. 9). Without analyte, LiZnVO4 modified glassy carbon electrode (LiZnVO4/GCE) didn’t show any voltammetric signature in the given potential window, signifying that LiZnVO4/GCE can be used as a sensor towards the nitrite detection. Curve (b) and (a) of Fig. 9 represent the characteristic oxidation peaks corresponding to the bare GCE and LiZnVO4/GCE in the presence of nitrite, respectively. Increased peak current with low potential was observed for LiZnVO4/GCE when compared to bare GCE indicating the electro catalytic effect of modified surfaces.
Furthermore, the oxidation peak of nitrite for the LiZnVO4/GCE was observed at 1.16 V. Peak current was found to be nearly 2 times greater than that of bare GCE, proposing the electrocatalytic movement of LiZnVO4 towards the oxidation of nitrite analyte, which can be accounted its unusual basic features and spectacular electrochemical properties [26].
6.1 Effect of pH
The impact of pH on peak current was examined over the pH range from 2 to 12.0 using phosphate buffer solutions, as shown in Fig. 10. Nitrite response was assessed using LiZnVO4/GCE in presence of 2 mL of 5 mM of nitrite solution. With extending pH, the current raises and extended to the utmost value at pH = 12. In this mode, the buffer solution of pH = 12 was chosen as the working medium for further studies. This shows that, strong basic condition was important to get a better current response due to decreased participation of protons in the electrode reaction. Similarly, it showed that the peak potential is also dependent on the pH.
6.2 Variation of scan rate: effects on nitrite oxidation
To know the impact of scan rate on peak currents was examined by conducting the cyclic voltamtery of 2 mL of 5 mM nitrite at various scan rates starting from 50 mVs−1 to 150 mVs−1 using LiZnVO4/GCE in the presence of buffer pH 12, as depicted in Fig. 11. In order to determine whether the electrochemical reaction is diffusion controlled or adsorption controlled, a plot of log of current versus log of scan rates was drawn (Fig. 12). From this graph, it is confirmed that the electrochemical reaction of nitrite is diffusion controlled [27] as its slope was found to be 0.4322 which is closer to 0.5 with the linear regression equation log (I) = 0.4322 log (scan rate) − 3.94906 having R2 value of 0.98031and the surface of modified GCE was not defiled by them.
6.3 Effect of concentration on electrochemical oxidation of nitrite
Electrochemical sensing of nitrite was further investigated by changing the concentration of nitrite from 0.5 μM to 5 μM by keeping the scan rate (50 mV/s) and pH buffer solution (pH = 12) as constant and is shown in the Fig. 13. Peak currents were extended moderately with the increment of nitrite concentration, as shown in Fig. 14. From this plot, a linear regression was obtained to be Ip = 3.00 C + 1.757 where Ip is in μA and C in μM, respectively. The limit of detection for nitrite detection was calculated to be 27.5 nM which is obtained from 3Sb/m, where Sb is the standard deviation for the 5 independent cyclic voltammetric response recorded for the blank solution. As the detection limit is too low, LiZnVO4 NPs based sensor can be used as a sensible candidate for the detection of nitrite.
6.4 Stabiltiy analysis
Stability of LiZnVO4/GCE towards the detection of nitrite was studied using 5 mM nitrite, KCl and pH 12 solution following the same procedure as described previously. The CV performance of LiZnVO4/GCE has been studied persistently for seven consecutive days. A plot of current vs. time is shown in Fig. 15. and inset shows the percentage of current retention. Almost constant currents is observed upto the 7th day and the variation of current was very small. Peak currents at 1st day and 7th day were found to be 3.09 × 10–5 and 2.99 × 10–5 A, respectively. Current retention was found to be 97% at 7th day. These results suggest that LiZnVO4 NPs based sensors are more stable towards the detection of nitrite.
7 Electrochemical supercapacitive behavior of LiZnVO4 NPs: Energy storage applications
The supercapacitive characterizations of the prepared materials have been studied using 3-electrode system subjected to various scan rates using cyclic voltammetric method. Figure 16a. shows the CV curves for the prepared LiZnVO4 electrode with varied scan rates. Scan rates were varied from 20 mVs−1 to 10,000 mVs−1. The system produced stabilized CV curves even at high scan rate of 10,000 mVs−1 and this high scan rate is uncommon and rarely reported in the literature [28].
The most widely utilized scan rates are below 1 V/s. Here, it is started with a 20 mVs−1 scan rate and a Cs was obtained to be 11.034 F/g corresponding to the discharge current (I) of 4.1 μA/g. Then the scan rate was increased to 50, 100, 200, 300, 400, 500 mVs−1 and the Cs’s were found to be 10.23, 8.41, 7.35, 6.65, 6.23 and 5.91, respectively. Cs was found to be 3.076 F/g at 10,000 mVs−1. At 20 mVs−1, the area of the CV curve was very small, as the scan rate increases; area of the CV curves also increases, without changing in the curve shape.
As shown in Fig. 16c, discharge currents increased linearly with increasing scan rate. It was initially 4.1, 8.55 and 14.5 μA/g for 20, 50 and 100 mVs−1 respectively. Later, it was raised to 208, 266, 311 and 532 μA/g for 3000, 4000, 5000 and 10,000 mVs−1, respectively. But Cs of the electrode decreases with increasing scan rate shown in Fig. 16d which is a familiar trend. At a very low scan rates, particles in the electrolyte have adequate time and the chance to enter the material through openings, however, they gather on the external surface of the electrode at higher scan rates.
Potentio-electrochemical impedance analysis (PEIS) was performed using Biologic EC-Lab model. Charge transfer resistance (RCT) was obtained from the diameter of the semicircle part of the spectrum and linear part of it reveals about the capacitance of the material (Fig. 16b). Z- fit analysis was performed to know the equivalent circuit for the obtained plot. RCT was found to be 117 Ohm, which was obtained from the difference of final and initial resistance of the semicircle. The equivalent circuit was found to be C1/(R1 + C2/R2 + M3) as shown in Fig. 17d, where C1, C2 are the capacitances, R1 and R2 are the resistances and M is the restricted diffusion.
Energy density of the LiZnVO4 coated electrode was found to be 8.83 mWhkg−1 with a Cs of 11.034 F/g and Cs at an ultra high scan rate of, 10,000 mVs−1 was found to be 3.1 F/g.
Electrochemical energy storage of LiZnVO4 NPs-based supercapacitor can be furthermore analysed by subjecting it to galvanostatic charge–discharge (GCD) technique at different current densities. The resultant capacitive performance was observed from E-T curves with current densities of 0.1, 0.15, 0.2 and 0.24 mA, as shown in Fig. 17a.
The Cs’s are acquired from the GCDs using Eq. (5) were found to be 88.7, 85, 79, 76.3 and 60.8 F/g at current densities of 0.1, 0.15, 0.2 and 0.24 mA, respectively, as shown in Fig. 17c. This shows the decrease of Cs’s with increasing in current rate.
where, Ed is the energy density and ∆t is the discharge time.
Cycling stability of LiZnVO4 NPs-based supercapacitor was examined at a current density of 0.24 mA (inset of Fig. 17b) by subjecting to 1000 cycles. A stable GCD curves were attained upto 1000 cycles. % of Capacity retention at 1000th cycle was found to be 92%, shown in Fig. 17b.
In supercapacitors and batteries, a Ragone diagram, a plot of power density and energy density, was plotted. It facilitates the assessment of electrochemical energy storage performance of various energy storage devices. Here, a ragone diagram was plotted to recognize the variation of power density and energy density corresponding to the different Cs’s of LiZnVO4 NPs-based supercapacitor obtained from several GCD’s, shown in the inset of Fig. 17c. Power density and energy density at a Cs of 88.7 F/g were found to be 0.51 W/g and 12.3 Wh/Kg, respectively.
All these features recommend that of LiZnVO4 NPs-based supercapacitor preserves the stability upto 1000 cycles of GCD’s and is therefore well suited for electrochemical pseudocapacitive energy storage applications.
8 Humidity sensing of LiZnVO4 NPs:
A cylindrical disc of LiZnVO4 NPs was made using hydraulic press. It has nearly 8 mm of diameter and a 1.5 mm width. Ohmic connections on either faces of the disc were made by painting the silver paste and its humidity sensing parameters were measured using indigenous experimental setup, shown in Fig. 18. The arrangement showing glass chambers containing various saturated solutions to maintain specific % relative humidity (%RH) and are monitored by humidity meter (Mextech-DT-615). Every glass chamber was connected to a holding apparatus along with a rubber cork in order to hold the disc. The other ends of the electrodes are connected to a digital multimeter (Hioki DT 4282, Japan) to measure its electrical resistance before and after exposure of the sample to a specific %RH at ambient temperature.
LiZnVO4 NPs was examined for the humidity sensing performance. Figure 19 shows a plot of resistance of LiZnVO4 versus % RH conditions. A resistance of 28 MΩ was obtained at 11.0%RH and this value found decreasing with the increase in %RH. At %RH of 97, resistance of LiZnVO4 was found to be 1 MΩ, which is extremely low. This significant drop in resistance enhances the extent of conductivity of LiZnVO4 because of the adsorption of water vapors on the sample surface due to the presence of open pores as revealed from its SEM image. The sensitivity factor (Sf) was calculated using the Eq. (6) and it was found to be 28. Using the method of the least square fit, the real sensitivity was calculated and found to be 0.3 MΩ/%RH. A correlation coefficient R2 = 0.997 was obtained. Also, variation of resistance of the sample with %RH showing an excellent linearity suggests the significant sensitivity of the material. Limit of detection (LOD) of the sample was determined using the standard error estimation method [29] and was found to be 5%RH. Such good sensitivity, linearity and low LOD signify that LiZnVO4 is well-structured material for fabrication of humidity sensors practicable at room temperature.
where, Ri is the initial resistance at 11%RH and Rf is the final resistance at 97%RH, respectively.
The falling in resistance of LiZnVO4 with relative humidity is owing to the Grotthuss chain mechanism illustrated in Fig. 20. As relative humidity increases from 11 to 95% RH, resistance of LiZnVO4 found decreased, which is a normal trend. This means the conductivity of the material increases, with an increase in RH at room temperature, is because of adsorption of H2O molecules on the planes of LiZnVO4 disc. Besides, these physisorbed water molecules undergoes ionization due to the strong electric field and high local charge density resulting in the production of hydronium ions (H3O+) in a greater extent and act as charge carriers by a sequence of exchange reactions. The presence of this type of protonic conductions in the adsorbed layers of water increases the conductivity and decreases the resistance drastically at higher RH [30].
Apart from sensitivity studies, studies on timing behavior of LiZnVO4 are crucial for designing an effective humidity sensor Fig. 21. Depicts the typical response and recovery curves of the LiZnVO4 at room temperature. The response and recovery times recorded for the LiZnVO4 sample were 20 s and 27 s respectively. A rapid timing performance was due to the presence of open pores on its surface for easy diffusion of water vapors, as discovered from its SEM studies. In addition, adsorption is spontaneous whereas desorption process takes an additional 7 s, as it requires extra energy to break the bonds formed between the surface of LiZnVO4 and water molecules adsorbed on it [31].
Stability of LiZnVO4 towards humidity sensing was examined by recording its resistance for every five days in all RH conditions, over a period of 1 month. From the Fig. 22, it was confirmed that LiZnVO4 NPs show superior sensing stability. This effective, stable performance offers an additional advantage which further promotes the development of LiZnVO4 based humidity sensors.
9 Conclusion
We have successfully synthesized LiZnVO4 NPs by employing the simple solution combustion method utilizing Hibiscus leaves as a combustible fuel. XRD assures the formation of the rhombohedral phenacite structure of the product. FT-IR strengthens the presence of the Zn–O and Li-VO4 bonds. The produced LiZnVO4 NPs exhibited significant antioxidant activity with the lowest IC50, 37.32 μg/mL. It has the advantages of developing latent fingerprints for crime investigations. LiZnVO4 NPs were used to fabricate green emission LED. It is fruitful in examining the applicability of the LiZnVO4 NPs based sensors in the electro analytic detection of nitrite at a lower concentration level. The specific capacitance and energy density of LiZnVO4 electrode was found to be 11.034 F/g and 8.83 mWhkg−1 at a scan rate of 20 mVs−1 and 88.7 F/g at a current density of 0.1 mA. Power density and energy densities were found to be 0.51 W/g and 12.3 Wh/Kg, respectively. Hence, LiZnVO4 NPs were also well suited for electrochemical pseudo-capacitive energy storage applications. Finally, it shows an excellent outcome of the humidity sensing of LiZnVO4 with a very high sensitivity factor implies higher the sensitivity of the material towards the moisture, making it an outstanding candidate for the fabrication of current humidity sensors. Therefore, LiZnVO4 NPs can be used for multiple applications such as, optical, biological, electrochemical and energy storage applications.
Data availability
All data obtained during the analysis are in original format and can be accessed upon reasonable request from the corresponding author.
References
Phaniendra, et al., Free radicals properties, sources, targets, and their implication in various diseases. Ind. J. Clinic Biochem 30(1), 11–26 (2015)
X. Luo et al., Application of nanoparticles in electrochemical sensors and biosensors. Electroanal. Int. J. Devoted Fund. Pract. Aspects Electroanal. 18(4), 319–326 (2006)
J.J. Sindelar et al., Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide 26(4), 259–266 (2012)
P.S. Adarakatti et al., Amino-calixarene-modified graphitic carbon as a novel electrochemical interface for simultaneous measurement of lead and cadmium ions at picomolar level. J Solid State Electrochem 20(12), 3349–3358 (2016)
L. Zeng et al., Hierarchical LiZnVO 4@ C nanostructures with enhanced cycling stability for lithium-ion batteries. Dalton Trans. 44(17), 7967–7972 (2015)
Y. Lin et al., A new anode material LiZnVO4: synthesis and electrochemical measurements. Ionics 19(3), 391–394 (2013)
D Guyomard, et al., New amorphous oxides as high capacity negative electrodes for lithium batteries the LixMVO4 (M= Ni, Co, Cd, Zn; 1< x≤ 8) series. Journal of power sources, 1997 68(2): 692–697
S. Khursheed et al., Effect of Synthesis Methods on Structural, Optical and Spectral Properties of Bluish-Green LiZnVO4 Phosphor. Materials Focus 5(5), 485–488 (2016)
G. Viacava et al., Antioxidant activity of butterhead lettuce: evaluation of significant factors affecting antioxidant extraction and quantification. J Food Meas Characterization 9(2), 206–214 (2015)
S. Kundu et al., A planar supercapacitor made of supramolecular nanofibre based solid electrolyte exhibiting 8 V window. Nano Energy 61, 259–266 (2019)
U. Holzwarth et al., The Scherrer equation versus the’Debye-Scherrer equation’. Nat Nanotechnol 6(9), 534–534 (2011)
K.S. Babu et al., Synthesis and optical characterization of porous ZnO. J. Adv. Ceram. 2(3), 260–265 (2013)
B. Grandhe et al., Spectral characterization of novel LiZnVO4 phosphor. Optics Communications 285(6), 1194–1198 (2012)
C.B. Rodella et al., V 2 O 5/TiO 2 catalytic xerogels Raman and EPR studies. J. sol-gel Sci. Technol. 25(1), 83–88 (2002)
R. Zamiri et al., Far-infrared optical constants of ZnO and ZnO/Ag nanostructures. RSC Adv. 4(40), 20902–20908 (2014)
H. Sasabe et al., High power efficiency blue-to-green organic light-emitting diodes using isonicotinonitrile-based fluorescent emitters. Chem. An Asian J. 12(6), 648–654 (2017)
H.J. Bolink et al., Green light-emitting solid-state electrochemical cell obtained from a homoleptic iridium (III) complex containing ionically charged ligands. Chem Mater 18(12), 2778–2780 (2006)
K. Jongseong et al., Phenothiazine derivatives as an easily accessible emitter for green light-emitting electrochemical cells. J. Luminescence 197, 383–388 (2018)
H.S. Yogananda et al., New design of highly sensitive and selective MoO3: Eu3+ micro-rods: Probing of latent fingerprints visualization and anti-counterfeiting applications. J. Colloid Inter. Sci. 528, 443–456 (2018)
E. Prabakaran et al., Nanomaterials for latent fingerprint detection: a review. J. Mater. Res. Technol. 12, 1856–1885 (2021)
P. Sharma et al., DPPH antioxidant assay revisited. Food Chem. 113(4), 1202–1205 (2009)
P. Ionita et al., Is DPPH stable free radical a good scavenger for oxygen active species. Chem Pap 59(1), 11–16 (2005)
M. Zamani et al., Scavenging performance and antioxidant activity of γ-alumina nanoparticles towards DPPH free radical: Spectroscopic and DFT-D studies. Spectrochimica Acta Part A 201, 288–299 (2018)
O.E. Adebiyi et al., In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia Beni-Suef University. J. Basic Appl. Sci. 6(1), 10–14 (2017)
A. Matuszewska et al., Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE 13(6), e0197044 (2018)
C. Wei et al., Simultaneous electrochemical determination of hydroquinone, catechol and resorcinol at Nafion/multi-walled carbon nanotubes/carbon dots/multi-walled carbon nanotubes modified glassy carbon electrode. Electrochim. Acta 149, 237–244 (2014)
A.A. Rafati et al., Simultaneous determination of ascorbic acid, dopamine, and uric acid using a carbon paste electrode modified with multiwalled carbon nanotubes, ionic liquid, and palladium nanoparticles. Microchim. Acta 181(15–16), 1999–2008 (2014)
T.M. Dinh et al., High-resolution on-chip supercapacitors with ultra-high scan rate ability. J. Mater. Chem. A 2(20), 7170–7174 (2014)
B. Chethan et al., Humidity sensing performance of hybrid nanorods of polyaniline-Yttrium oxide composite prepared by mechanical mixing method. Talanta 215, 120906 (2020)
T. Divya et al., Nanoceria based thin films as efficient humidity sensors. Sens. Actuat A: Phys 261, 85–93 (2017)
Chethan, et al., Polypyyrole based core-shell structured composite based humidity Sensor operable at room temperature. Sens. Actuators B 296, 1266 (2019)
Acknowledgements
Soundarya T L indebted to Principal, CEO and Director, Siddaganga Institute of Technology for extending laboratory and characterization facilities. G Nagaraju thanks to DST Nanomission, Govt. of India, New Delhi for financial support (No.SR/NM/NS-1262/2013). Authors also thank DST-SAIF, KOCHI and Center of Applied Research and nano-Technology, S.I.T, Tumakuru for TEM and SEM analysis.
Author information
Authors and Affiliations
Contributions
Material development, data collection, data analysis, writing were performed by TLS. Editing and reviewing by CU. Humidity sensing its analysis was performed by YTR. BN and GN Research administration.
Corresponding authors
Ethics declarations
Conflict of interest
All authors affirm that they have no compete of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soundarya, T.L., Udayabhanu, Ravikiran, Y.T. et al. Green synthesis of LiZnVO4 nanoparticles and its multiple applications towards electrochemical sensor, supercapacitor, humidity sensing, photoluminescence and antioxidant activities. J Mater Sci: Mater Electron 33, 10902–10918 (2022). https://doi.org/10.1007/s10854-022-08070-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-08070-7