Abstract
Recycled polypropylene/polyaniline/graphene nanoplatelets (rPP/PANI/GNPs) nanocomposites were fabricated via ultrasonic-assisted single-screw extruder at 150–170 °C with a rotating screw speed of 50 rpm. The ultrasonic wave frequency and power supply were kept constant at a frequency of 20 kHz and 6 kW, respectively. The composition of the polymer nanocomposites used in this study was 92 wt.% rPP and 8 wt.% PANI, denoted as rPP/PANI. The effects of GNPs loadings (0.5, 1.5, and 3.0 parts per hundred resin (phr)) on the structural, morphological, thermal, and electrical properties on the nanocomposites were systematically evaluated. The X-ray diffraction (XRD) and fourier transform infrared spectroscopy (FTIR) showed the presence of GNPs characteristics at 26.5°, 42.40°, 54.51°, and interactions between GNPs and rPP/PANI nanocomposites at different GNPs loadings. The compatibility of GNPs in rPP/PANI nanocomposites was confirmed by the morphological study, which showed to an enhancement in the electrical properties of the nanocomposites. The results also showed that the incorporation of 3 phr GNPs into rPP/PANI nanocomposites resulted in a lower degree of crystallinity of about 20.8% and a higher electrical conductivity of about 4.1 × 10–1 S cm−1. The current work paves a way towards understanding how to effectively enhance the electrical conductivity of rPP/PANI nanocomposites using GNPs, leading to potential use in electronic applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polypropylene (PP) is one of the most extensively used polyolefins due to its economical, recyclable, and good processability characteristics [1,2,3]. The widespread usage of PP may lead to considerable waste which will cause negative environmental impacts; hence, rPP has been explored as a way to create new products inexpensively or at low production cost [4]. Apart from having low thermal stability and mechanical properties [4], rPP is also an insulator which shows that it has low electrical conductivity; this limits its usage in high-performance applications such as sensors, supercapacitors, actuators, etc. [5]. Therefore, a conducting polymer like PANI can be added to rPP in order to meet the requirements for engineering applications including supercapacitors, sensors, and membranes (used in water and wastewater treatment) [6, 7]. Recently, polymer nanocomposites filled with PANI have gained popularity because of their synergistic effects due to the enhanced processability of the conventional polymers and the high electrical conductivity (σ) of PANI [6, 8]. A new nanocomposite system was developed by mixing rPP and PANI; this nanocomposite exhibits a combination of their properties, i.e., insulating rPP and conducting PANI, thereby expanding its usage in various applications. Attractive features such as low cost, easy synthesis, high conductivity, and good environmental stability make PANI one of the conducting polymers most extensively used with thermoplastic polymers [5, 7]. Furthermore, Cho et al. [6] reported that the PANI nanofiber can significantly improve the σ of the PP-based nanocomposites.
Recently, GNPs have emerged as the most promising reinforcing nanofiller because of its extraordinary properties [9, 10]. GNPs are also known as graphite nanoplatelets, graphite nanosheets, graphite nanoflakes, and exfoliate or expanded graphite [9]; they consist of multiple stacked graphene layers and exist in the planar structure [11]. The combination of properties such as low cost, superior mechanical properties, good thermal and electrical conductivity, and high aspect ratio makes GNPs attractive and ideal as nanofillers to improve the properties of polymer nanocomposites [1, 9, 12]. Numerous researches have utilized GNPs as nanofillers in PP [1, 2, 12, 13] or PANI [14,15,16] composites, respectively. Generally, it was found that the incorporation of GNPs enhances the thermal and electrical properties of polymer nanocomposites [17,18,19]. For example, Li et al. [13] prepared PP/GNPs nanocomposites via simple solvent dispersion method and observed an enhancement in thermal stability and electrical conductivity with increasing loadings of GNPs. Besides that, Badi et al. [15] fabricated nanocomposite film using the spin-coating technique of GNPs and PANI, and found that the presence of GNPs significantly improved the electrical conductivity of the nanocomposite compared to pure PANI. However, the effects of GNPs on the properties of rPP/PANI composites have not been adequately reported.
In this study, rPP/PANI/GNPs nanocomposites were prepared by means of ultrasound-assisted single-screw extrusion process. The influence of GNPs loading on the structural, morphological, thermal, and electrical conductivity of rPP/PANI/GNPs nanocomposites was systematically investigated. To the best of our knowledge, there is limited literature review on the same polymer nanocomposite system fabricated using the same method, i.e., mixing using ultrasonic-assisted single-screw extruder. Hence, this work is essential to provide a basic understanding on developing conductive polymeric nanocomposites for future applications such as energy storage, sensors, electrical, and electrochemical devices.
2 Experimental
2.1 Materials
rPP was purchased from Titan Petrochemical, Malaysia. Aniline (ANI) and ammonium persulfate (APS) with 99% and 98% purity were bought from Sigma-Aldrich (M) Sdn. Bhd., respectively. The molecular weights of aniline and APS were 93.13 g/mol and 228.20 g/mol, respectively. Hydrochloric acid, HCl 37% was procured from Qrec (Asia) Sdn. Bhd.. Dicumyl peroxide (DCP) with a molecular weight of 270.37 g/mol was bought from Acros Organics, New Jersey, USA. GNPs (Grade: GNP-M-5, 99.5% carbon) was bought from XG Sciences (East Lansing, MI, USA). The average diameter and thickness of GNPs were 15 µm and ≤ 10 nm, respectively.
2.2 Nanocomposites preparation
Prior to rPP/PANI/GNPs nanocomposite fabrication, PANI was self-synthesized using ultrasonic-assisted chemical oxidative polymerization method with APS as oxidant and HCl as dopant. The synthesis of PANI was carried out based on the steps described in our previous research [20]. Subsequently, rPP, PANI, DCP, and GNPs were mixed using an ultrasonic-assisted single-screw extruder. The ultrasound probe was located at the die section of the extruder barrel and a 6 kW ultrasonic power supply was connected to the die zone to generate ultrasonic waves at a frequency of 20 kHz. The tip of the ultrasonic horn came into contact with the molten compound. The composition of rPP (92 wt.%), PANI (8 wt.%), DCP (1 phr- parts per hundred of resin), and GNPs (0.5, 1.5, and 3 phr) were used in the present research. Then, the extrudate was pelletized and dried prior to being molded into small pellet forms (7 mm diameter and 1 mm thickness). The process and proposed mechanism for the synthesis of rPP/PANI/GNPs nanocomposites are clearly illustrated in Figs.1 and 2.
Referring to Fig. 2, it was proposed that the rPP will interact with the PANI in the presence of DCP as an initiator. Subsequently, the added GNPs will interact with PANI via hydrogen bonding and π–π interactions. Hence, the combination of all these interaction results in good interfacial adhesion between rPP/PANI and GNPs.
2.3 Characterizations
The crystallinity of the samples was characterized by X-ray diffraction (XRD, D8 Bruker Advance Diffractometer) with CuKα radiation (λ = 1.541 Å) as radiation source (40 kV, step size 0.05, and a counting time of 50 s per step) in the range of 2θ from 5 to 60°. The functional groups of the samples were determined by Fourier transform infrared spectroscopy (FTIR, PerkinElmer) under the transmittance mode in the range of 400–4000 cm−1. The morphological and microstructural characterizations of the samples were accomplished by field emission scanning electron microscopy (FESEM, Nova NanoSEM 450). The melting temperature, crystallization temperature, and melting enthalpy were determined by using differential scanning calorimetry (DSC) analysis (Perkin Elmer DSC-6). The samples was heated from 30 to 350 °C at 10 °C/min heating rate, and subsequently held for 10 min prior to cooling at a similar rate of 10 °C/min. The degree of crystallinity, Xc (in percentage), were calculated based on the equation: \({\text{X}}_{{\text{c}}} { }\left( {\text{\% }} \right) = {{\Delta {\text{H}}_{{\text{m}}}^{*} } \mathord{\left/ {\vphantom {{\Delta {\text{H}}_{{\text{m}}}^{*} } {{\text{W}}_{{\text{f}}} \times \Delta }}} \right. \kern-\nulldelimiterspace} {{\text{W}}_{{\text{f}}} \times \Delta }}{\text{H}}_{{\text{m}}}^{{\text{o}}} \times { }100\), where \({\Delta \mathrm{H}}_{\mathrm{f }}^{\mathrm{o}}\) is the melting enthalpy of fully crystalline PP (209 J/g) [21], \({\Delta \mathrm{H}}_{\mathrm{m}}^{*}\) is the experimental melting enthalpy of rPP/PANI/GN nanocomposites, and \({\mathrm{W}}_{\mathrm{f}}\) is the weight fraction of rPP in rPP/PANI/GN nanocomposites.
A multifunction digital four-point probe tester (Model: ST2258C, Jiangsu, China) was used to measure the conductivity of rPP/PANI/GN nanocomposites. The electrical conductivity (σ, S cm−1) measurements were performed at room temperature. A total of five-time measurements were taken on each composite pellet and the average values of conductivity were reported.
3 Results and discussion
3.1 Structural analysis
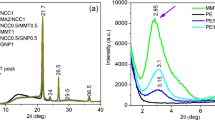
XRD patterns of rPP/PANI, rPP/PANI/GNPS nanocomposites, and GNPs are presented in Fig. 3a, b. In the XRD pattern for pure GNP, a single high-intense diffraction peak can be observed at 2θ = 26.5° with an inter-spacing (d) of 3.35 Å, corresponding to the XRD peak of the (022) crystalline plane of the GNPs. The peaks at 42.40°, 54.51° as 2θ were ascribed to the GNPs with crystalline planes of (100), and (004), respectively [22,23,24]. In the XRD pattern (Fig. 3a), four distinctive peaks of rPP can be observed at 16.51°, 18.32°, 21.52°, and 28.1° corresponding to crystalline planes of (040), (130), (131), and (220), whereas four PANI peaks appeared at 13.90° and 25.21° associated to crystalline planes of (112) and (122), respectively [25,26,27,28]. In the XRD pattern for rPP/PANI/GNPs nanocomposites, peaks corresponding to both rPP/PANI and GNPs were visible. Furthermore, the sharpest peak intensities of rPP ((040) and (130)) and PANI (112) were reduced in the range of 13° ≤ θ ≤ 20° (Fig. 3c), whereas the sharpest peak of GNPs increased in the range of 26° ≤ θ ≤ 27° (Fig. 3d) with increasing GNPs loading. More specifically, the reduction of these peaks in the XRD patterns indicates the decreased crystallinity of rPP and PANI which was due to the increase in GNPs content. The crystallite size was calculated by debye–scherrer equation [29] and found to have experienced a reduction with increasing GNPs. The crystallite sizes of rPP were (40 nm, 37 nm, 35 nm, 33 nm) and PANI were (38 nm, 36 nm, 35 nm, 30 nm) for rPP/PANI/GNPs nanocomposites with 0 GNPs, 0.5 GNPs, 1.5 GNPs, and 3.0 GNPs, respectively. However, when the GNPs loading was increased, the growth of the rPP/PANI composite was somewhat suppressed which led to the degradation of rPP/PANI composite peak intensity, thus reducing the crystallite size [30].
FTIR spectra of pure rPP/PANI and rPP/PANI/GNPs nanocomposites are presented in Fig. 4. For pure rPP/PANI, the characteristic peaks in the range of 3000–2800 cm−1 corresponded to the C-H stretching vibration in CH3, CH2, and CH groups of rPP [31, 32]. The other peaks that belong to rPP were observed at 1468 cm−1 (asymmetric bending of CH3), 1374 cm−1 (symmetric bending of CH3), 1162 cm−1 (C–C stretching, C–H bending, and CH3 rocking), and 998–975 cm−1 (C–C stretching and CH3 rocking) [31,32,33].
Meanwhile, the characteristic peaks located at 3424 cm−1 and 1560 cm−1 belong to the amine N–H stretching and C=C stretching vibration of PANI quinoid structure, respectively [5, 8, 33, 34]. Additionally, the characteristic peak at 1641 cm−1 is assigned to the quinone stretching deformation of PANI [20]. However, it can be observed that most of the characteristic peaks of PANI overlap with the characteristic peaks of rPP. For instance, the peaks in the range of 3000–2800 cm−1, 1468 cm−1, 1374 cm−1, and 1162 cm−1 indicate the C–H stretching, C=C stretching of the benzenoid rings, C–N stretching, and in-plane C–H bending of quinoid structure of PANI, respectively [5, 8, 35, 36].
Figure 4 shows the presence of a new characteristic peak at 2358 cm−1 with the addition of GNPs to rPP/PANI nanocomposites at all GNPs loadings. This characteristic peak belongs to GNPs and was due to the C–O vibration from C–OH [37].
Moreover, with increasing GNPs loadings, the characteristic peaks intensity at 3424 cm−1 (O–H stretching vibration at the edge of the GNPs plane), 3000–2800 cm−1 (C=O of GNPs), 1560 cm−1 (C=C stretching of quinoid rings), 1468 cm−1 (C=C stretching of benzenoid rings), 1374 cm−1 (C–N stretching), and 1162 cm−1 (in-plane C–H bending of quinoid structure) were found to have increased. This could be due to the hydrogen bonding and π–π interactions which developed between GNPs and PANI in the composites [36, 37]. It appears that the interaction between GNPs and PANI enhances the degree of electron delocalization, thereby increasing the conductivity of rPP/PANI/GNPs nanocomposites.
3.2 Morphological analysis
The morphology of rPP/PANI and rPP/PANI/GNPs nanocomposites were illustrated by FESEM analysis at different magnifications of 5000× and 25,000× (Fig. 5). As illustrated in Fig. 5a, rPP/PANI nanocomposites show a rough surface morphology with the existence of voids which reveals the insufficient adhesion between rPP and PANI. However, the introduction of GNPs in rPP/PANI (Fig. 5b–g) showed the presence of good interfacial adhesion between GNPs and rPP/PANI as indicated by the smaller amount of voids observed on the surface of the nanocomposites. The GNPs were found to have embedded and fill the voids which were a result of the poor adhesion between rPP and PANI. Furthermore, with increasing GNPs loading up to 3 phr (Fig. 5f), there was an absence of large agglomerates suggesting that the interaction between GNPs and PANI hindered the restacking and agglomeration of GNPs and enhanced its compatibility with rPP/PANI nanocomposites. GNPs have disordered structures formed by aggregated thin and crumbled nanosheets, large specific surface area, and high surface energy which can provide more electron transfers in order to enhance the electrical conductivity of the material [22].
3.3 Thermal and electrical analysis
In electronic devices and their applications, one of the vital criteria is the thermal performance of the fabricated material composites, which is greatly related to the material’s composition and microstructure [38]. The thermal performance of rPP/PANI/GNPs nanocomposites with different loadings of GNPs were studied using DSC analysis. Table 1 provides the DSC results of the melting temperature (Tm), crystallization temperature (Tc), melting enthalpy (\({\Delta \mathrm{H}}_{\mathrm{m}}^{*}\)), and crystallinity percentage (Xc) of rPP/PANI/GNPs nanocomposites at different GNPs loadings. It is noted that the increase in GNPs loading in rPP/PANI/GNPs nanocomposites has an insignificant effect on the Tm of rPP. As Tm is largely dependent on the forms of crystals present, this result indicates that the crystal structure of rPP remained consistent with the addition of all loadings of GNPs. GNPs were observed to have caused β-crystal formation in PP composites that are characterized by a secondary melting peak between 150 and 160 °C, but in insignificant amounts which do not affect the Tm [39, 40]. However, a small amount of GNPs (i.e., 0.5 phr) was found to increase the Tc of rPP in the rPP/PANI/GNPs nanocomposites from 119.2 to 123.5 °C, with an increment of about 3.3 °C. Additionally, Tc further increased with increasing GNPs loading up to 3 phr. This could be due to the fact that GNPs induced the crystallization of rPP in rPP/PANI/GNPs nanocomposites via heterogeneous nucleation effect. Altay et al. [21] also reported that the primary reason for the enhancement in Tc of PP-based composites with increasing graphene content is attributed to the heterogeneous nucleation by graphene. Table 1 also shows that the Tc of rPP/PANI/3.0GNPs is smaller than that of rPP/PANI/1.5GNPs (only by 0.1 °C), which might be caused by some impurities included during the conducting of DSC analysis [41].
However, the Xc of rPP in rPP/PANI/GNPs nanocomposites decreased with the addition of GNPs, and further decreased with increasing GNPs loading. Although the presence of GNPs induced crystallization, it restricted the chain mobility of rPP; hence it does not facilitate crystal growth during the crystallization process. As a result, the overall crystalline regions formed in the rPP/PANI/GNPs nanocomposites were reduced thereby resulting in a decrease in the crystallization of rPP. Similar findings have been reported by Inuwa et al. [42], who observed a decrease in the Xc of PP in polyethylene terephthalate (PET)/PP/GNPs composites with increasing GNPs loading. Additionally, crystallinity and electrical conductivity of rPP/PANI/GNPs nanocomposites are correlated. A decrease in the crystalline region helps to raise the conductivity of composites since the electrical conductivity increases with an increase in the amorphous regions [43].
Table 2 demonstrates the electrical conductivity (σ) of the raw rPP, PANI, GNPs, and rPP/PANI nanocomposites as a function of GNPs loading. Table 2 shows that the σ of rPP alone was 1.3 × 10–16 S cm−1, in which the value at a power scale of 10–11 is classified as an insulator [44, 45]. However, the σ of rPP was greatly increased with the addition of PANI to a value of about 2.25 × 10–8 S cm−1. The value of σ for rPP/PANI nanocomposites was found to increase to about 5.6 × 10–6 S cm−1 with the addition of low levels of GNPs loading (i.e., 0.5 phr). Moreover, the σ value of rPP/PANI/GNPs nanocomposites was found to increase with increasing GNPs loading up to 3 phr. For instance, the values of σ for rPP/PANI nanocomposites with 1.5 phr and 3 phr of GNPs loadings were 7.6 × 10–4 S cm−1 and 4.1 × 10–1 S cm−1, respectively. The σ values with the power scale ranging from 10–9 to 10–1 S cm−1 can be grouped as the semiconductors [44, 45]. Therefore, this finding represents the transition of rPP/PANI nanocomposites from a low conductive semiconductor (2.25 × 10–8 S cm−1) to a highly conductive semiconductor (4.1 × 10–1 S cm−1), with increasing GNPs up to 3 phr. The improvement in the σ of rPP/PANI nanocomposites with increasing GNPs loading could be attributed to a few reasons. One of them is the high σ of GNPs. A similar observation was reported by Modak et al. [46] who claimed that higher concentrations of GNPs provide better networking for electrical conduction due to the high σ of GNPs. Furthermore, the increases in the physical contact between the dispersed GNPs-PANI chains tend to create a conductive network in the rPP/PANI nanocomposites. Subsequently, more electrons are able to move or jump directly between the conductive materials, leading to an increase in the σ of the composites [45, 47]. Another reason for the enhancement in σ can be explained by an increase in the amorphous regions of the rPP/PANI/GNPs nanocomposites.
Krause et al. [48] synthesized PP/GNPs nanoparticles via melt compounding using twin-screw extruder. An excellent electrical conductivity of 3.03 × 10–7 S cm−1 was achieved for 7.5 vol.% of GNPs loading. Meanwhile, Li et al. [13] studied the effect of GNPs on the electrical conductivity of PP/GNPs particles. An electrical conductivity of 6.25 × 10–5 S cm−1 was obtained for 15 wt.% GNPs loading. In addition, an electrical conductivity of 0.13 S cm−1 was achieved in Badi et al. [15] research for PANI/GNPs synthesized via direct mixing methods at room temperature. However, this research showed an excellent enhancement in electrical conductivity (4.1 × 10–1 S cm−1) for the combination of rPP/PANI and GNPs.
4 Conclusion
rPP/PANI/GNPs nanocomposites were successfully fabricated using ultrasonic-assisted single-screw extruder. The XRD and FTIR results confirmed the preparation of rPP/PANI/GNPs nanocomposites and also indicated a reduction in crystallinity with the addition of GNPs. Morphological results showed that less voids appeared on the rPP/PANI nanocomposites surface upon the addition of GNPs. DSC results clearly implied that the addition of higher GNPs loading reduced the crystallinity of rPP/PANI/GNPs nanocomposites. The electrical conductivity increased as the GNPs loading increased. The maximum electrical conductivity (4.1 × 10–1 S cm−1) was attained in rPP/PANI nanocomposites with 3 phr of GNPs. It was determined that rPP/PANI/GNPs nanocomposite may be a new ideal conductive material for advanced electronic applications through the incorporation of GNPs.
References
M.G. Lee, S.W. Lee, J.H. Cho, J.Y. Jho, Improving dispersion and mechanical properties of polypropylene/graphene nanoplatelet composites by mixed solvent-assisted melt blending. Macromol. Res. 28, 1166–1173 (2020)
J.Z. Liang, J.Z. Wang, G.C.P. Tsui, C.Y. Tang, Thermal properties and thermal stability of polypropylene composites filled with grapheme nanoplatelets. J. Thermoplast. Compos. Mater. 31(2), 246–264 (2018)
A.L. Pang, H. Ismail, Tensile properties, water uptake and thermal properties of polypropylene/waste pulverized tire/kenaf (PP/WPT/KNF) composites. BioRes. 8(1), 806–817 (2013)
K. Zdiri, A. Elamri, M. Hamdaoui, O. Harzallah, N. Khenoussi, J. Brendle, Reinforcement of recycled PP polymers by nanoparticles incorporation. Green Chem. Lett. Rev. 11(3), 296–311 (2018)
A.M. Rahnamol, J. Gopalakrishnan, Improved dielectric and dynamic mechanical properties of epoxy/polyaniline nanorod/in situ reduced grapheme oxide hybrid nanocomposites. Polym. Compos. 41(8), 2998–3013 (2020)
S.H. Cho, M.K. Kim, J.S. Lee, J.S. Jang, Polypropylene/polyaniline nanofiber/reduced graphene oxide nanocomposite with enhanced electrical, dielectric, and ferroelectric properties for a high energy density capacitor. ACS Appl. Mater. Interfaces 7(40), 22301–22314 (2015)
F.X. Perrin, C. Oueiny, Chapter 5-Polyaniline-based thermoplastic blends, ed. By P.M. Visakh, C.D. Pina, E. Falletta, (Elsevier, 2018), pp. 117–147
M.E. Ali Mohsin, N.K. Shrivastava, A. Arsad, N. Basar, A. Hassan, The effect of pH on the preparation of electrically conductive and physically stable PANI/sago blend film via in situ polymerization. Front. Mater. 7(20), 1–11 (2020)
S. Paszkiewicz, A. Szymczyk, Z. Rosłaniec, Chapter 5-Graphene derivatives in semicrystalline polymer composites, ed. by A. Tiwari, M. Syvajarvi, (Scrivener Publishing LLC, 2016) pp. 145–146
D.D. Yang, H.P. Xu, W. Yu, J.R. Wang, X.C. Gong, Dielectric properties and thermal conductivity of graphene nanoplatelet filled poly(vinylidene fluoride) (PVDF)/poly(methyl methacrylate) (PMMA) blend. J. Mater. Sci. Mater. Electron. 28, 13006–13012 (2017)
P.N. Khanam, M.A. AlMaadeed, M. Ouederni, E. Harkin-Jones, B. Mayoral, A. Hamilton, D. Sun, Melt processing and properties of linear low density polyethylene-graphene nanoplatelet composites. Vacuum 130, 63–71 (2016)
M.A. Al-Saleh, A.A. Yussuf, S. Al-Enezi, R. Kazemi, M.T. Wahit, T. Al-Shammari, A. Al-Banna, Polypropylene/graphene nanocomposites: effects of gnp loading and compatibilizers on the mechanical and thermal properties. Mater. 12(23), 3924–3934 (2019)
Y.F. Li, J.H. Zhu, S.Y. Wei, J.E. Ryu, L.Y. Sun, Z.H. Guo, Poly(propylene)/graphene nanoplatelet nanocomposites: melt rheological behaviour and thermal, electrical, and electronic properties. Macromol. Chem. Phys. 212(18), 1951–1959 (2011)
B. Abad, I. Alda, P. Dıaz-Chao, H. Kawakami, A. Almarza, D. Amantia, D. Gutierrez, L. Aubouy, M. Martın-Gonzalez, Improved power factor of polyaniline nanocomposites with exfoliated graphene nanoplatelets (GNPs). J. Mater. Chem. A. 1(35), 10450–10457 (2013)
N. Badi, S. Khasim, A.S. Roy, Micro-Raman spectroscopy and effective conductivity studies of graphene nanoplatelets/polyaniline composites. J. Mater. Sci. Mater. Electron. 27, 6249–6257 (2016)
S. Khasim, Polyaniline-graphene nanoplatelet composite films with improved conductivity for high performance X-band microwave shielding applications. Results Phys. 12, 1073–1081 (2019)
A.R. Ravindran, C. Feng, S. Huang, Y. Wang, Z. Zhao, J. Yang, Effects of graphene nanoplatelet size and surface area on the ac electrical conductivity and dielectric constant of epoxy nanocomposites. Polym. 10(5), 477–493 (2018)
T. Evgin, A. Turgut, G. Hamaoui, Z. Spitalsky, N. Horny, M. Micusik, M. Chirtoc, M. Sarikanat, M. Omastova, Size effects of graphene nanoplatelets on the properties of high-density polyethylene nanocomposites: morphological, thermal, electrical, and mechanical characterization. Beilstein J. Nanotechnol. 11, 167–179 (2020)
A. Shubha, S.R. Manohara, Effect of graphene nanoplatelets concentration on optical, dielectric and electrical properties of poly(2-ethyl-2-oxazoline)-polyvinylpyrrolidone-graphene nanocomposites. J. Mater. Sci. Mater. Electron. 31, 16498–16510 (2020)
M.R. Husin, A. Arsad, A. Hassan, O. Hassan, Influence of different ultrasonic wave on polymerization of polyaniline nanofiber. Appl. Mech. Mater. 618, 50–54 (2014)
L. Altay, M. Atagur, K. Sever, I. Sen, T. Uysalman, Y. Seki, M. Sarikanat, Synergistic effects of graphene nanoplatelets in thermally conductive synthetic graphite filled polypropylene composite. Polym. Compos. 40(1), 277–287 (2019)
U. Mehmood, H. Asghar, F. Babar, M. Younas, Effect of graphene contents in polyaniline/graphene composites counter electrode material on the photovoltaic performance of dye-sensitized solar cells (DSSCSs). Sol. Energy 196, 132–136 (2020)
B. Jiang, B. Peng, A. Zhu, C. Zhang, Y. Li, Eco-friendly synthesis of graphene nanoplatelets via a carbonation route and its reinforcement for polytetrafluoroethylene composites. J. Mater. Sci. 53, 626–636 (2018)
S. Gutic, A.S. Dobrota, N. Gavrilov, M. Baljozovic, I.A. Pasti, S.V. Mentus, Surface charge storage properties of selected graphene samples in ph-neutral aqueous solutions of alkali metal chlorides-particularities and universalities. Int. J. Electrochem. Sci. 11, 8662–8682 (2016)
I. Karacan, H. Benli, An X-ray diffraction study for isotactic polypropylene fibers produced with take-up speeds of 2500–4250 m/min. Tekstil Ve Konfeksiyon. 21, 201–209 (2011)
B.S. Rao, N. Maramu, E.V. Rao, N.S. Rao, K.R. Prasad, Deconvolution of x-ray diffraction spectrum of polypropylene. Res. Rev. J. Phys. 2(3), 1–4 (2018)
M. Zhang, X. Wang, T. Yang, P. Zhang, X. Wei, L. Zhang, H. Li, Polyaniline/graphene hybrid fibers as electrodes for flexible\supercapacitors. Synth. Met. 268, 116484 (2020)
S. Palsaniya, H.B. Nemade, A.K. Dasmahapatra, Synthesis of polyaniline/graphene/MoS2 nanocomposite for high performance supercapacitor electrode. Polym. 150, 150–158 (2018)
M. Ahmadipour, M.J. Abu, M.F.A. Rahman, M.F. Ain, Z.A. Ahmad, Assessment of crystallite size and strain of CaCu3Ti4O12 prepared via conventional solid-state reaction. Micro. Nano. Lett. 11(3), 147–150 (2016)
D. Zheng, H. Yang, F. Yu, B. Zhang, H. Cui, Effect of graphene oxide on the crystallization of calcium carbonate by C3S carbonation. Mater. 12, 2045–2054 (2019)
I. Raut, M. Calin, Z. Vuluga, E. Alexandrescu, M.L. Arsene, V. Purcar, C.A. Nicolae, A.M. Gurban, M. Doni, L. Jecu, Comparative study on the behavior of virgin and recycled polyolefins–cellulose composites in natural environmental conditions. J. Compos. Sci. 3(2), 60–74 (2019)
S.A. Stoian, A.R. Gabor, A.M. Albu, C.A. Nicolae, V. Raditoiu, D.M. Panaitescu, Recycled polypropylene with improved thermal stability and melt processability. J. Therm. Analy. Calorim. 138, 2469–2480 (2019)
M. Kılıc, U. Alkan, Y. Karabul, H.B. Yamak, O. Icelli, The effects of PANI concentration on the mechanical properties of PP/PANI composites. AKU J. Sci. Eng. 18(2), 426–433 (2018)
M.H.M. Moghadam, S. Sabury, M.M. Gudarzi, F. Sharif, Graphene oxide-induced polymerization and crystallization to produce highly conductive polyaniline/graphene oxide composite. J. Polym. Sci. Part A Polym. Chem. 52(11), 1545–1554 (2014)
M. Mitra, C. Kulsi, K. Chatterjee, K. Kargupta, S. Ganguly, D. Banerjee, S. Goswamid, Reduced graphene oxide-polyaniline composites-synthesis, characterization and optimization for thermoelectric applications. RSC Adv. 5(39), 31039–31048 (2015)
Q. Wang, Y.M. Wang, Q.G. Meng, T.L. Wang, W.H. Guo, G.H. Wu, L. You, Preparation of high antistatic HDPE/polyaniline encapsulated graphene nanoplatelet composites by solution blending. RSC Adv. 7(5), 2796–2803 (2017)
O.A. Al-Hartomy, S. Khasim, A. Roy, A. Pasha, Highly conductive polyaniline/graphene nano-platelet composite sensor towards detection of toluene and benzene gases. Appl. Phys. A. 125, 12–20 (2019)
M.S. Gumaan, Chromium improvements on the mechanical performance of a rapidly solidifed eutectic Sn-Ag alloy. J. Mater. Sci. Mater. Electron. 31, 10731–10737 (2020)
Y.S. Jun, J.G. Um, G. Jiang, A. Yu, A study on the effects of graphene nano-platelets (GnPs) sheet sizes from a few to hundred microns on the thermal, mechanical, and electrical properties of polypropylene (PP)/GnPs composites. eXPRESS Polym. Lett. 12(10), 885–897 (2018)
E. Watt, M.A. Abdelwahab, M.R. Snowdon, A.K. Mohanty, H. Khalil, M. Misra, Hybrid biocomposites from polypropylene, sustainable biocarbon and graphene nanoplatelets. Sci. Rep. 10, 10714–10726 (2020)
M.A. Yusof, N.H. Nor Rahman, S.Z. Sulaiman, A.H. Sofian, M.S.Z. Mat Desa, I. Izirwan, Development of low density polyethylene/graphene nanoplatelets with enhanced thermal properties. IEEE 9th International Conference on Mechanical and Intelligent Manufacturing Technologies, 6-9 (2018)
I.M. Inuwa, A. Hassan, S.A. Shamsudin, Thermal properties, structure and morphology of graphene reinforced polyethylene terephthalate/polypropylene nanocomposites. Malays. J. Analy. Sci. 18(2), 466–477 (2014)
A.H. Mohamad, O.G. Abdullah, S.R. Saeed, Effect of very fine nanoparticle and temperature on the electric and dielectric properties of MC-PbS polymer nanocomposite films. Results Phys. 16, 102898–102906 (2020)
J. Pionteck, Chapter 1- Introduction, Handbook of antistatics, ed. By J. Pionteck, G. Wypych, (Elsevier, 2017) pp. 1–15
M.A. Tarawneh, S.A. Saraireh, R.S. Chen, S.H. Ahmad, M.A.M. Al-Tarawni, M. Al-Tweissi, L.J. Yu, Mechanical, thermal, and conductivity performances of novel thermoplastic natural rubber/graphene nanoplates/polyaniline composites. J. Appl. Polym. Sci. 137(28), 48873–48883 (2020)
P. Modak, S.B. Kondawar, D.V. Nandanwar, Synthesis and characterization of conducting polyaniline/grapheme nanocomposites for electromagnetic interference shielding. Procedia Mater. Sci. 10, 588–594 (2015)
D.R. Dhakal, P. Lamichhane, K. Mishra, T.L. Nelson, R.K. Vaidyanathan, Influence of graphene reinforcement in conductive polymer: synthesis and characterization. Polym. Adv. Technol. 30(9), 2172–2182 (2019)
B. Krause, P. Rzeczkowski, P. Potschke, Thermal conductivity and electrical resistivity of melt-mixed polypropylene composites containing mixtures of carbon-based fillers. Polym. 11(6), 1073–1087 (2019)
Acknowledgements
The authors would like to thank Universiti Teknologi Malaysia for the research Grants (R.J130000.7751.4J497, R.J130000.7844.4F288, and Q. J130000.21A2.05E25).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pang, A.L., Husin, M.R., Arsad, A. et al. Effect of graphene nanoplatelets on structural, morphological, thermal, and electrical properties of recycled polypropylene/polyaniline nanocomposites. J Mater Sci: Mater Electron 32, 9574–9583 (2021). https://doi.org/10.1007/s10854-021-05620-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-05620-3