Abstract
ZnWO4:Eu3+ red nanophosphors have been synthesized by a simple environmentally friendly and low-cost molten salt method. The phase structure, morphology, SAED, photo-luminescent (PL), lifetime, color rendering, quantum efficiency and stability properties of the phosphor were investigated in detail. The XRD results indicated that all Eu3+ ions doping ZnWO4 samples crystallize in a single crystal wolframite structure and exhibit superior crystallinity. Under the excitation of 394 and 465 nm, ZnWO4:Eu3+ phosphors can be exhibited red light emission. The photoluminescence excitation spectra of ZnWO4:Eu3+ phosphors show several emission peaks, on account of the f–f transitions of Eu3+ ions. The quantum efficiency of ZnWO4:0.07Eu3+ phosphors were calculated to be 40.5%, the CIE chromaticity coordinate of ZnWO4:0.07Eu3+ phosphors were (0.631, 0.364) closed to the red phosphors standard value (0.670, 0.330), based on the Judd–Ofelt analysis, Judd–Ofelt intensity parameters (Ω2/Ω4) includes symmetry of Eu3+ ions crystal field environment, it was suggested that ZnWO4:0.07Eu3+ phosphors were a potential phosphor in LEDs. This research sheds new light on improving the quantum efficiency, stability properties and CIE chromaticity coordinate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of society, people pay great attention to the luminescent material that to strengthen the luminescent properties, a lot of literatures have reported [1] that rare earth ions doping have important applications in luminescent properties. In the tungstate materials [2], Zinc tungstate is special, it is different from other materials, which belongs to a kind of self-activating phosphor, ZnWO4-based materials are divided into single crystal and nanoparticles and so on [3, 4]. Tungstate materials have attracted intensive attention originating from typical merits, such as high light yield, high average refractive index, physical, chemical, and structural properties, which has been widely used in lighting and display devices, including plasma display panels, white light-emitting diodes (WLEDs) [5]. Up to now, one of the main obstacles is improving the stability properties, which can be extremely difficult for its development and commercial in WLEDS.
There are several methods were prepared for the rare earth ions doping in improving the luminescent properties of the phosphor. The literature has reported that Dang Yuan et al. A novel electrochemical sensor for the selective determination of hydroquinone and catechol using synergic effect of electropolymerized nicotinic acid film and Cd-doped ZnWO4 nanoneedle [6], Minzhu Zhao et al. Investigation of energy transfer mechanism and luminescent properties in Eu3+ and Sm3+ co-doped ZnWO4 phosphors [7], Chen Guiqiang et al. Improving red emission by co-doping Li+ in ZnWO4:Eu3+ phosphors [8], Chai Xiaona et al. Upconversion luminescent and temperature-sensing properties of Ho3+/Yb3+-codoping ZnWO4 phosphors based on luminescent intensity ratio [9], Chunyang Li et al. Color changing from white to red emission for ZnWO4:Eu3+ nanophosphors at a different temperature [10], therefore, in this work, the nanocrystals of ZnWO4:Eu3+ phosphors have been prepared by the molten salt method.
As it can be observed in the literature [11], Eu3+ ions doping frequently play important roles in morphologies as well as in luminescent properties of phosphors. At the same time, it was used for an activator by the rare earth ions doping the phosphors. The purpose of this work are used to investigate the luminescent properties of phosphors, Judd–Ofelt [12] the theoretical analysis, quantum efficiency and stability properties. So far, to the best of our knowledge, there has not yet been any report similar to our work. For the first time, the goal of this work is discussed in detail for the luminescent properties of phosphors and Judd–Ofelt analysis. The contributions of this paper can be summarized as follows better understand luminescent properties, quantum efficiency and stability properties of the sample.
2 Experimental methods
2.1 Preparation of ZnWO4 and ZnWO4:Eu3+ specimen
All chemical reagents are analytical reagent (AR) and from Sinopharm Chemical Reagent Co., Ltd. China. All chemicals were utilized without further purification. In brief, all specimens operated at a fixed doping concentration of the Eu3+ ions was 7 mol%, Eu3+ ions doping ZnWO4 nanophosphors were prepared via the molten salt method. Using analytically pure (AR) Na2WO4·2H2O (99.5%), Eu2O3 (99.99%) and Zn(CH3COO)2·2H2O (99%) as raw materials and LiNO3 (99%)–NaNO3 (99%) as molten salt media. The raw materials Na2WO4·2H2O and Zn(CH3COO)2·2H2O were weighed out according to the composition of ZnWO4. The molten salt with the weigh ratio about LiNO3:NaNO3 to 1:3 and dissolved. Then, the raw materials were added to the molten salt solution. The mixture of raw materials and molten salt (molar ratio of 1:4), and then placed in a mortar to grind for 30 min, subsequently, the mixture was heated to the target temperature (180 °C) at a speed rate of 5 °C/min and kept for 8 h in the room temperature. After cooling, the solidified melt was washed with distilled water to remove the sodium salt and residual lithium salt. The silver nitrate solution was not tested until a white precipitate was produced, which demonstrated that there was no excess Cl− in the solution. Ultimately, the obtained specimens were dried at 60 °C for analysis.
2.2 Material characterization
The structure of obtained powders were characterized by X-ray diffraction patterns (XRD, D/max, 2200 PC) at a 2θ range from 10° to 70° with 0.02°/step size with Cu-Kα radiation (λ = 1.5405 Å). Rietveld refinement of the sample was presented by the General Structure Analysis System software (GASA). The morphologies of samples were observed by field emission scanning electron microscopy (Q45, FEI, America). UV–Vis–NIR spectrophotometer (Cary5000, Agilent, America) was used to record the absorption spectra in the range of 200–800 nm. The Raman spectrum was tested by a microscopic confocal laser spectrometer (Renishaw-invia, Renishaw, Britain). The light source was the laser of an argon ion laser with a spectral resolution of 2 cm−1. XPS (X-ray photoelectron spectroscopy, Axis Supra, Britain) was measured the composition of the elements and oxygen vacancy of the sample. The excitation spectra and emission spectra were detected by Photoluminescence spectrometer (F-4600, Hitachi, Japan) and the decay time was obtained by using the xenon lamp as an excitation source. The quantum efficiency of the phosphors were conducted with a Quantum-QY Plus UV–NIR absolute PL quantum yield spectrometer (C9920-03, Hamamatsu photonics K.K., Japan) with samples in powder form and the temperature-dependent PL spectra were evaluated on Hitachi FLS-980 Fluorescence Spectrophotometer. All the above measurements were performed out at room temperature.
3 Results and discussion
3.1 XRD characterization
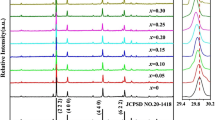
The XRD spectra of ZnWO4 phosphor and ZnWO4:0.07Eu3+ crystal particles were obtained by the LTMS method in LiNO3–NaNO3 at 8 h. As shown in Fig. 1, it can be observed all the diffraction peaks can be perfectly indexed to monoclinic crystal structured ZnWO4 (JCPDS card No. 15-0774), which demonstrates that the substitution of Eu3+ ions have a little modest effect on the crystal structured of ZnWO4. Furthermore, under the same synthesis condition, all the observed diffraction peaks of the ZnWO4 and ZnWO4:0.07Eu3+ phosphors are strong and sharp, which implies good crystallinity and observes from the diffraction pattern, this paper found no other secondary and mixed phase in the figure. Therefore, all prepared samples are the monoclinic wolframite structure, as can be observed from the partial enlarge from 28º to 33º of the figure. Obviously, it can be noticed that the diffraction peaks of the diffraction pattern shift toward a lower angle deviation due to the Eu3+ ions doping which was described to expand the lattice. According to the similar ionic radius and stability of the crystal system, the ion radius of the Eu3+ (0.95 Ǻ) is close to the ionic radius of Zn2+ (0.74 Ǻ), however, the larger than the W6+ (0.60 Ǻ) ions radius. Therefore, the Eu3+ ions will preferentially replace the Zn2+ position in the ZnWO4 matrix. Based on the Bragg equation:
where d is the interplanar distance of corresponding crystal plane, θ is the diffraction angle, λ is the wavelength of the X-ray, where n is the number of reflection orders (generally speaking the value of the n is 1), it is interestingly found that with the reduction of the diffraction angle, interplanar distance of corresponding crystal plane becomes larger. According to the above analysis, this may be because the Eu3+ ions preferentially replace the Zn2+ position in the ZnWO4 lattice, which lead to an increase in the spacing between the crystal faces. The analysis of these results suggests that the sharp and high diffraction peaks with the higher crystallinity of the phosphor. From the crystal structure diagram of the ZnWO4 of the view, ZnO6 and WO6 octahedrons are linked to each other by shared oxygen, it can easily conclude that the introduction of Eu3+ entered into the ZnWO4 which could bring defects and increase the oxygen vacancies of the surface [13].
3.2 TEM analysis and EDS spectrum analysis
Figure 2 exhibits the SEM images of the (a), HRTEM images of the (b), SAED (c) of the ZnWO4:0.07Eu3+ phosphors. The sample has a homogeneous morphology with a diameter of the 7–20 nm which content with the pattern of the XRD. The ZnWO4:0.07Eu3+ phosphors with rod-like morphology due to the higher aspect ratio, to clearly understand the crystallinity and purity of the sample by observed the HRTEM. As can be observed in Fig. 2b, the average gap is the lattice fringe of 0.48 and 0.545 nm corresponding to the lattice plane of the (100) and (010) respectively, which further confirmed the formation of the monoclinic structure of wolframite ZnWO4. The SAED figures were shown in Fig. 2c. The picture is consists of a few laps, it is generally accepted that the samples were polycrystalline, it is composed of a lattice, therefore it was concluded that the specimen of a single crystal.
3.3 XPS analysis
To obtain further information on the analysis chemical composition of elements and surface state of the ZnWO4:0.07Eu3+ nanorods, the XPS measurements were displayed in Fig. 3. The survey spectrum as illustrated in Fig. 3a, indicating the presence of Zn 2p, W 4f, O 1s, Eu 3d in the as-obtained sample without other elemental signals being observed. With carbon C 1s peak Tag the binding energies (BE) [14]. The XPS peak of C 1s is due to accidental hydrocarbons from the XPS measurement. As can be observed in Fig. 3b, the BE values of C 1s is 284.8 eV, it suggests that the doped Eu3+ is trivalent. In the XPS spectrum of Zn 2p, two characteristic peaks located at 1021.88 and 1044.98 eV (Fig. 3c) can be assigned to Zn 2p3/2 and Zn 2p1/2, respectively. However, for the pure ZnWO4, Zn 2p3/2 and Zn 2p1/2 are centered at 1021.72 and 1044.94 eV. The XPS spectrum of W 4f exhibits two peaks that occurred in 35.38 and 37.48 eV (Fig. 3d), originating from the W 4f7/2 and W 4f5/2 of W6+, respectively. Compared with pure ZnWO4, it can easily conclude that there is a shift of 0.63 eV and 0.67 eV to the high binding energy. As displayed in Fig. 3e, crystal lattice oxygen was observed in the peak at 530.88 eV in the Eu3+ doping ZnWO4 nanocrystal. In comparison with the pure ZnWO4 phosphor, the BE value of O 1s is 530.71 eV which in accordance with the holding peak of the O2− ion. For the XPS spectrum of Eu 3d, the main two peaks at the binding energies of 1126 and 1155 eV can be ascribed to the Eu 3d5/2 and Eu 3d3/2 of Eu3+ (Fig. 3f) [15]. The energy of the peaks is 17,145 eV which originated from the Eu 3d3/2 of the ZnWO4 which in good agreement with the previous work [16]. Based on the above-mentioned analysis results, it can be concluded that the valence C, Zn, W, O and Eu elements are 0, + 2, + 6, − 2, + 3, respectively.
3.4 The ultraviolet–visible reflectance absorption spectra analysis and optical band gap energy analysis
The band structure was conducted by ultraviolet–visible reflectance absorption spectra to investigate the intrinsic electronic of ZnWO4:0.07Eu3+ nano-rods (Fig. 4a). In comparison with the band structure of the pure ZnWO4 with the same synthesis, the condition was depicted in Fig. 4a. As can be seen in the picture, Eu3+ ions doping the ZnWO4 can strengthen the optical response of the ZnWO4 phosphors, the band gap energy of the phosphors, which the equation is expressed as follows [17]:
where α is absorption coefficient, B is the characteristic constant of relating to the material, where hν is the photon energy, h is Planck's constant (h = 4.14 × 10–15 eVs). Eg is the optical band gap energy, t is the exponent associated with electron transitions, where t = 1/2 is for directly allowed transition, t = 2/3 means directly prohibit transitions, t = 2 represents indirect transitions are allowed, t = 3 stands for indirect forbidden transition, from the literature [18], the optical transition of ZnWO4 is directly allowed, therefore where the value of t is 0.5, as presented in Fig. 4b. On the basis of the above Eq. (2), the optical band gap of pure ZnWO4 and ZnWO4:0.07Eu3+ phosphors were estimated to be 3.18 and 3.16 eV, respectively. As expected, the band gap values of ZnWO4:0.07Eu3+ phosphors were lower than of the pure ZnWO4 phosphor, indicating that the as-prepared ZnWO4:0.07Eu3+ phosphors have a higher intrinsic electronic conductivity, and may be demonstrated that the superior electrochemical performances. In general, owing to rare earth ions doping, resulted in the impurity levels are formed, the result suggests that Eu3+ ions enter into the ZnWO4 host. Confirming the ZnWO4:0.07Eu3+ phosphors have a higher absorptive capacity than the ZnWO4 host. In this figure, strong absorption band was observed at 350–490 nm, it was obviously observed that have one broad absorption band locating at 350–490 nm, which shows strong absorption ability located in 465 nm, at the same time, there is an obvious absorption peak, which point on the absorption spectrum corresponds to the 465 nm, deriving from the characteristic transition (7F0 → 5D2) of the Eu3+ [19].
3.5 Raman spectrum analysis
The structure of ZnWO4 phosphor was further analyzed using the Raman spectrum, which is an effective way of studying the vibrational modes of lattices and molecules, there are a series of literatures [20] have reported that vibrational modes of ZnWO4 contain 36 patterns. The Raman spectrum is classified into external vibration and internal vibration mode, Raman activity parameters is Ag and Bg, and the optical. This can be formulated as follows:
where g is Raman activity parameters, the Raman spectra of the ZnWO4 was connected with other reports [21], and the pure peak can be observed prominent absorptions in the spectral range of 100–1000 cm−1 which correspond to six characteristic vibration patterns in Fig. 5. The bands at ~ 588 and 719 cm−1 are linking to symmetrical stretching vibrations connecting O atom in Zn–O–W [22], the two bands nearby 424 and 468 cm−1 are depicting into asymmetric deformation of Zn–O and W–O in ZnO6 and WO6 octahedral ligand [23]. The two bands located at ~ 875 and 826 cm−1 were originating from the stretching pattern of WO6 octahedron [24]. Raman spectrum was also completed to comprehend the influence of the doping. As displayed in Fig. 5, the relevant reports show the Raman shifts of the ZnWO4 at 123, 141, 162, 190, 276, 320, 340, 405, 515, 542, 677, 709, 785 and 903 cm−1, which explains this task. The three Raman bands located at ~ 123, 190 and 542 cm−1 can be described into the symmetric stretching of the ZnO6 octahedron clusters, at the same time, the rest of the bands are a feature of the vibration pattern of WO6 octahedron group. Vibration patterns are divided into internal and external modes. The internal extension patterns of WO6 octahedron group were expressly solved as six Raman bands for the A1g symmetric extension (~ 903 cm−1). The Eg (709 and 785 cm−1) and T2g (2Ag + Bg, ~ 405, 340 and 190 cm−1) were corresponding to asymmetric stretching and bending deformation of W–O bonds. The Raman band located at ~ 162 cm−1 was ascribed from the inter-chain torsion and distortion, however, that at ~ 276 cm−1 might be named as distortion vibration (Ag) of cationic sub-lattice of WO6 octahedron group [25]. In addition, bands in the 500–600 cm−1 range are connected with W–O–W symmetric extension and the other located at ~ 141, 320 and 677 cm−1 are corresponding to the external vibration pattern of WO6 octahedron cluster movement against Zn2+ [26]. The pure rod-like ZnWO4 shows the strongest Raman, due to larger aspect ratio, hence led to the stronger Raman peaks [27]. Furthermore, the flexural vibration of the WO6 octahedron group (190 cm−1), locating at ~ 190 cm−1 originating from the symmetric extension vibration of ZnO6 octahedron group, which verified that pure ZnWO4 host with higher lattice symmetry, because no other defects were created ZnWO4 the crystal structure. As shown in figure, the samples have to turn into the high band, when the Eu3+ occupy the Zn2+ of the ZnWO4 host lattice, resulting in the distortion of the lattice. That could be caused by the small lattice and the offset of the Raman. In the internal vibration modes, Zn–O bonds are lower than the W–O bonds of the WO6 octahedron group.
3.6 Photoluminescence (PL) analysis
The excitation spectrum of ZnWO4:0.07Eu3+ phosphors were obtained by monitoring the emission wavelength at 616 nm, as displayed in Fig. 6a. The emission spectra can be observed the characteristic emission peaks of the Eu3+ [28], located in 594, 616, 654 and 705 nm, which originated from the transition of Eu3+, corresponding to the transition of 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, 5D0 → 7F4, respectively [29]. Electron transition 5D0 → 7F2 is predominant, when the transition intensity of the 5D0 → 7F2 higher than the transition intensity of the 5D0 → 7F1, suggesting the Eu3+ ions occupy the asymmetric site of the lattice and no inversion center. Under the 616 nm emission spectra, in the wavelength wide range of 340–500 nm, it can be found that the suitable amount of purity in the products may produce some defects to become the new luminescent center, which is favorable for the luminescence of ZnWO4:0.07Eu3+ phosphors.
Figure 6b displays the emission spectra of ZnWO4:0.07Eu3+ phosphors under the excitation of 394 and 465 nm. It is interestingly to find the strongest intensity that was observed under 465 nm excitation, as shown in figure, all samples consist of the characteristic peak of the Eu3+, the peaks center at 594 and 616 nm, originating from the 5D0 state, and can be assigned to the magnetic dipole transition of Eu3+ (5D0 → 7F1) [30] and electric dipole transition of Eu3+ (5D0 → 7F2) [31], respectively. The weaker peaks at 654 nm, caused by the characteristic transition of the Eu3+ ions (5D0 → 7F3) [16], the peak located at 705 nm which was assigned as the characteristic transition of the Eu3+ (5D0 → 7F4).
3.7 Lifetime analysis
The single exponential fitting decay curve of ZnWO4:0.07Eu3+ phosphors were illustrated in Fig. 7. All decay life curves could be well fitted into first order exponential decay model function, and the single exponential fitting decay can be calculated by following formula [32]:
where I(t) is fluorescent intensity at time t, I(0) is the background intensity, A is the constant, τ is the lifetime, the single exponential decay model of the phosphor was mainly due to Eu3+-doped phosphors have same coordination environment into the host lattice and only exist in one deactivation process, the decay lifetime of the phosphor is 0.64 ms.
3.8 Electronic energy level scheme analysis
Figure 8 shows schematic diagram of energy level transitions of Eu3+ under excitation of 394 nm, excited by ultraviolet light 394 nm, Eu3+ ions were excited from 7F1 to 5D2 level and released to 5D0 state were called the way of the non-radiative process. Eu3+ absorbed the photon energy from the ground state return to 5L6, the energy was absorbed by the phosphors which emit non-radiative process will be dispersed to the crystal lattice. When 5D0 has dwelled, Eu3+ ions display efficient visible emissions because of the multi-channel transitions. The sensitivity or the effect of ligand ion strongly on the emission intensity of the 5D0 → 7F2 transition is a target to study the local site symmetry of Eu3+ ions in the crystal lattice. Based on the above analysis, the symmetry site was occupied by the Eu3+ ions, therefore the electric dipole transition suggests that Eu3+ ions were located at an asymmetric site without an inversion center. There are peaks located at 594, 616, 655 and 702 nm that corresponding to the radiative transition of the 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3 and 5D0 → 7F4 respectively. The corresponding photon energy can be obtained by calculating the photon energy equation [33]:
where E is the photon energy, h is the Planck's constant, ν is the frequency of light, c is the vacuum speed of light, the value of c is 3.0 × 108 m/s, λ is excitation and emission wavelength. The energy of the non-radiative transition was released up to 1.01 eV.
3.9 The quantum efficiency (QE) and thermal stabilities of the phosphor analysis
The quantum efficiency is one of a key parameter used to be estimated the practical application, under the 394 nm excitation, the quantum efficiency ZnWO4:0.07Eu3+ phosphors were measured with a Quantum-QY Plus UV–NIR absolute PL quantum yield spectrometer (C9920-03, Hamamatsu photonics K.K., Japan) with samples in powder form, and reference sample is BaWO4 was illustrated in Fig. 9. The instrument is composed of photonic multi-channel analyzer PMA-12 (the detector) and PLQY measurement software-PMA as well as the photonic multi-channel analyzer (C10028) with the addition of supplementary units, which contains an excitation laser unit and a filter unit (A10094). The test measurements were measured at room temperature. The quantum efficiency of the phosphors can be obtained as will see in the following the formula [34]:
N(Abs) respects the number of photons absorbed of the sample, where N(Em) is the number of photons emitted from a sample, λ is the wavelength, h is Planck’s constant and the value of h is 6.63 × 10–34 J·s, c is the velocity of light and the value is equal to 3 × 1017 nm/s, \(I_{{{\text{ex}}}}^{{{\text{sample}}}}\) represents the integrated intensities of the excitation light with sample, however, \(I_{{{\text{ex}}}}^{{{\text{reference}}}}\) means integrated intensities of the excitation light without a sample, \(I_{{{\text{em}}}}^{{{\text{sample}}}}\) and \(I_{{{\text{em}}}}^{{{\text{reference}}}}\) are the photoluminescence intensities with and without a sample, respectively. Based on the above test, the Quantum efficiency value of ZnWO4:0.07Eu3+ phosphors were calculated to be 40.5%. In recent years, for most of the phosphors, Quantum yield of phosphors were further improved in practical applications via adjusting the reaction conditions and the amount Eu3+ ions doping.
Figure 10 depicts thermal stabilities of the ZnWO4:0.07Eu3+ phosphors, the thermal stability is an important parameter for phosphors in WLEDs. Generally speaking, the temperature of the phosphors have an influence on thermal stability, attaching to its great influence on the efficiency of the phosphors. Integrated intensity of Eu3+ ions as a function of increasing temperature were depicted in Fig. 10. The integrated intensity of ZnWO4:0.07Eu3+ phosphors gradually declines, when the temperature changes from 180 to 300 °C, and dramatically decreases above 220 °C, it was mainly due to the thermal quenching, then it remains 90% of its initial intensity at 220 °C and reserves 85% at 240 °C. To further analysis the phosphor's thermal stability, the activation energy can be calculated by the Arrhenius equation as follows [35]:
where I0 and IT represents the initial luminescence intensity and intensity at testing temperature and at different room temperature, respectively. D denotes a constant for a certain matrix, k is the Boltzmann constant and the value of k is 8.629 × 10–5 eVK−1, Ea is activation energy of the thermal quenching, based on the above Eq. (7), the activation energy Ea of ZnWO4:0.07Eu3+ phosphors were calculated to be 0.578 eV. In comparison with CaGd4F14:Ce3+ phosphor and Y2MoSiO8:Eu3+ phosphor. According to the literature [36, 37], the activation energy (Ea) of CaGd4F14:Ce3+ phosphor and Y2MoSiO8:Eu3+ phosphor are 0.503 and 0.29 eV, respectively. ZnWO4:0.07Eu3+ phosphors have higher thermal stability, suggesting that it has better potential for indoor application of WLED [38].
3.10 Color coordinates analysis
Under the control of annealing temperature, upon the excitation by near ultraviolet light CIE chromaticity coordinates of the Eu3+-doped ZnWO4 phosphors were studied in Fig. 11. It is generally recognized that the color purity was calculated by the coordinate weighted average value relative to the light source point and the main control wavelength point, the formula is as follows [39]:
where (x, y) is color coordinate of phosphor light source, (xi, yi) is standard white light source color coordinates, (xd, yd) is the main emission coordinate of the emission spectrum, correlation color temperature is also an important parameter to measure the quality of light source, correlation color temperature was described as the following equation [40]:
where the value of (xe, ye) is (0.3320, 0.1858), (x, y) represents color coordinates of phosphors. The parameters of the above were represented, the relevant result was summarized as follows in Table 1. In this work, it is well known that it has important applications in the white light field of solid-state lighting. CIE standard white light source is the (0.33, 0.33). In this paper, color coordinate of ZnWO4:0.07Eu3+ phosphors were calculated is (0.631, 0.364) which is close to that of the NTSC standard CIE chromaticity coordinate value for (0.67, 0.33) and better than that of the commercial red Y2O2S:Eu3+, the color coordinates of ZnWO4:0.07Eu3+ phosphors are closer to the standard red light region. Therefore, in comparing with doping or un-doping, it is interestingly found that the color purity of the phosphor was improver than un-doping the ZnWO4 host. It was suggested that ZnWO4:0.07Eu3+ phosphors were applied in red-emitting phosphor as the next candidate for WLED.
3.11 Judd–Ofelt theory analysis
The theory can be used to explain the relationship between transition and relative intensity, which has become a powerful tool to study the luminescent properties of ions doping in solid-state lighting, it also provides information about the structural environment of metal ions and the chemical bonds of ions. According to the literature [41], the electric dipole transition intensity can be calculated the value of strength parameters Ωλ (λ = 2, 4, 6), there are two transition modes for Eu3+-doped the phosphors, one pattern is magnetic dipole transition of the 5D0 → 7F1, another mode is electric dipole transition, the magnetic dipole transition probability was expressed as follows [12]:
The Smd mean magnetic dipole line oscillator strength, the value of Smd is 7.83 × 10–42 esu2 cm2, it is a constant and isn't change with the substrate, where n is index of refraction of the host, h is Planck constant, ξ is average number of transitions, 5D0 → 7FJ corresponding to the electric dipole transition (J = 2, 4, 6), the radiative transition probability was calculated by following formula [42]:
where e is the unit charge, ɛ is the refraction factor and the value of ɛ is ε = n(n2 + 2)2/9, < ψJ||Uλ||ψ′J′ > 2 represents reduce the square of the matrix elements. The data of reduced matrix element was listed in Table 2. On the basis of the uniqueness of Eu3+, strength parameters Ωλ originated from 5D0 → 7Fλ (λ = 2, 4, 6), therefore A(JO) shown by the following:
The ratio of the emission peak area corresponding to the transition of the 5D0 → 7FJ (J = 2, 4, 6) to the emission peak area corresponding to the transition from 5D0 → 7F1, which is the intensity ratio of electric and magnetic dipole transitions as following equation [43]:
As mentioned in figure, the transition of the 5D0 → 7F6 was not observed in the emission spectrum, so the value of the 5D0 → 7F6 transition is equal to zero, radiation lifetime τ, lifetime contains radiative transition probability and non-radiative transition probability [44], the equation is shown as follows:
where AR is the radiative transition, AR = ∑AJ (J = 0, 1, 2, 3, 4), the quantum efficiency was calculated as follows [45]:
The ratio of η is radiative transition probability to the total radiative transition probability. The stimulated cross-sectional area (σe) of the sample was studied, it's a measure which used to judge the laser performance of materials, the formula is expressed as follows [46]:
λp means the wavelength of emission peak, C is for the speed of light, Δλeff is the effective bandwidth, the effective bandwidth is shown the equation [47]:
Table 3 shows some J–O parameters of Eu3+ ions in the diverse host, in comparison with the context, it can also be observed that the quantum efficiency of our powders were higher than other matrices, which indicated the radiative transition probability of Eu3+ ions in ZnWO4 phosphors were larger. Therefore ZnWO4:Eu3+ phosphors may manifest the higher luminescent efficiency.
Based on the theoretical calculation, when the temperature is 180 °C, the intensity parameter Ω2 value of ZnWO4:Eu3+ phosphors are 3.509 × 10–20 cm2, it can be observed the intensity parameters of ZnWO4:0.07Eu3+ crystal continues to increase. At the same time, the intensity parameter Ω4 of ZnWO4:Eu3+ phosphors are 1.080 × 10–20 cm2, respectively, the transition of 5D0 → 7F4 is very weak in the spectra. It is concluded that Ω4 is insensitive to the symmetry of its surroundings. All parameters were calculated in Table 3. The higher quantum efficiency indicates that the phosphors have better luminescent properties.
All relevant parameters were collected in Table 4, it can be seen that the excitation cross-sectional area corresponding to the electric dipole transition from 5D0 → 7F2 of phosphors had a larger value. The larger stimulated cross-section area is more favorable for the laser with high gain, the luminescent performance of the phosphors are longer and better. Indicating that the transition of 5D0 → 7F2 provides a good laser effect, more widely have potential applications in the optical display devices.
4 Conclusion
In this paper, we propose molten salt method-based hydrothermal reaction methods which makes principled use of Eu3+ doping. The Eu3+-doped ZnWO4:0.07Eu3+ phosphors have been successfully fabricated by the molten salt method. Under the excitation of 394 and 465 nm, the phosphors can be effectively excited. By introducing the Eu3+ ions into the ZnWO4 matrix, quantum efficiency can be obtained enhance. It is usually accepted that the intrinsic characteristics have influence on the fluorescent properties. Based on the above calculated results, the chromaticity coordinates of ZnWO4:0.07Eu3+ phosphors are (0.631, 0.364), the chromaticity coordinates of standard red phosphor is (0.67, 0.33), which suggests that ZnWO4:0.07Eu3+ phosphors locate in the red region, on the basis of calculating the J–O parameters (Ω2 > Ω4), denoting that Eu3+ ions occupy the lowest symmetric of the phosphor. To the best of our knowledge, we are the first to use the molten salt method for studying quantum efficiency and stabilities properties in ZnWO4:0.07Eu3+ phosphors. Therefore ZnWO4:0.07Eu3+ phosphors can become a potential phosphor in WLEDs.
References
R.S. Yadav, S.J. Dhoble, S.B. Rai, Enhanced photoluminescence in Tm3+, Yb3+, Mg2+ tri-doped ZnWO4 phosphor: three photon upconversion, laser induced optical heating and temperature sensing. Sens. Actuators B 273, 1425 (2018)
S. Mamidi, R. Gundeboina, K. Sreenu, M. Vithal, Urea-modified ZnWO4 with enhanced photocatalytic activity. J. Aust. Ceram. Soc. 54, 671 (2018)
M.T. Li, T. Takei, Q. Zhu, B.-N. Kim, J.-G. Li, Morphology tailoring of ZnWO4 crystallites architectures and photoluminescence of the doped RE3+ ions (RE = Sm, Eu, Tb, and Dy). Inorg. Chem. 58, 9432 (2019)
M. Mohamed Jaffer Sadiq, S. Mutyala, J. Mathiyarasu, D. Krishna Bhat, RGO/ZnWO4/Fe3O4 nanocomposite as an efficient electrocatalyst for oxygen reduction reaction. J. Electroanal. Chem. 799, 102 (2017)
Z. Xia, J. Zhuang, L. Liao, Novel red-emitting Ba2Tb(BO3)2Cl: Eu phosphor with efficient energy transfer for potential application in white light-emitting diodes. Inorg. Chem. 51, 7202 (2012)
Y. Dang, X.J. Wang, R.R. Cui, S.L. Chen, Y.Z. Zhou, A novel electrochemical sensor for the selective determination of hydroquinone and catechol using synergic effect of electropolymerized nicotinic acid film and Cd-doped ZnWO4 nanoneedle. J. Electroanal. Chem. 834, 196 (2019)
M.Z. Zhao, Y. Liu, S.Y. Ma, D. Liu, K. Wang, Investigation of energy transfer mechanism and luminescence properties in Eu3+ and Sm3+ co-doped ZnWO4 phosphors. J. Lumin. 202, 57 (2018)
G. Chen, F. Wang, J. Yu, H.S. Zhang, X. Zhang, Improved red emission by codoping Li+ in ZnWO4: Eu3+ phosphors. J. Mol. Struct. 1128, 1 (2017)
X.N. Chai, J. Li, X.S. Wang, Y.X. Li, X. Yao, Upconversion luminescence and temperature-sensing properties of Ho3+/Yb3+-codoped ZnWO4 phosphors based on fluorescence intensity ratios. RSC Adv. 7, 40046 (2017)
C.Y. Li, X.D. Du, D. Yue, M.N. Wang, J.B. Huang, Z.L. Wang, Color changing from white to red emission for ZnWO4: Eu3+ nanophosphors at different temperature. Mater. Lett. 171, 27 (2016)
R. Zhua, Y.S. Zeng, S.C. Liang, Y. Zhang, Y.H. Qi, Y.F. Liu, Y.N. Lyu, Regulated morphology of ScF3: Eu3+, Bi3+ microcrystals: microwave assisted hydrothermal synthesis, structure and luminescence properties. J. Solid State Chem. 269, 447 (2019)
Z.W. Zhou, W.W. Li, J.H. Song, B.C. Mei, G.Q. Yi, Y. Yang, Application of Judd–Ofelt theory in analyzing Nd3+ doped SrF2 and CaF2 transparent ceramics. J. Eur. Ceram. Soc. 39, 2446 (2019)
B.-W. Xun, Y.-C. Tang, J.-Y. Chen, B.-P. Zhang, Enhanced resistance in Bi(Fe1-xScx)O3–0.3BaTiO3 lead-free piezoelectric ceramics: facile analysis and reduction of oxygen vacancy. J. Eur. Ceram. Soc. 39, 4085 (2019)
L.B. Chang, G.Q. Zhu, Q.-U. Hassan, B.W. Cao, S.P. Li, Y.F. Jia, J.Z. Gao, F.C. Zhang, Q.Z. Wang, Synergetic effects of Pd0 metal nanoparticles and Pd2+ ions on enhanced photocatalytic activity of ZnWO4 nanorods for nitric oxide removal. Langmuir 35, 11265 (2019)
V.V. Atuchin, E.N. Galashov, O.Y. Khyzhun, A.S. Kozhukhov, L.D. Pokrovsky, V.N. Shlegel, Structure and electronic properties of ZnWO4 (010) cleaved surface. Cryst. Growth Des. 11, 2479 (2011)
Y.-M. Pan, W. Zhang, Z.-F. Hu, Z.-Y. Feng, L. Ma, D.-P. Xiong, P.-J. Hu, Y.-H. Wang, H.-Y. Wu, L. Luo, Synthesis of Ti4+-doped ZnWO4 phosphors for enhancing photocatalytic activity. J. Lumin. 206, 267 (2019)
G.-T. Xiong, W. Zhang, Z.-F. Hu, P.-J. Hu, Y.-M. Pan, Z.-Y. Feng, L. Ma, Y.-H. Wang, L. Luo, Photocatalytic activity of ZnWO4 phosphors doped with Li impurities. J. Lumin. 206, 370 (2019)
R.F. Gonçalves, E. Longo, A.P.A. Marques, M.D.P. Silva, L.S. Cavalcante, I.C. Nogueira, I.M. Pinatti, P.F.S. Pereira, M.J. Godinho, Structural investigation and photoluminescent properties of ZnWO4: Dy3+ nanocrystals. J. Mater. Sci. Mater. Electron. 28, 15466 (2017)
Y.Q. Zhai, M. Wang, Q. Zhao, J.B. Yu, X.M. Li, Fabrication and luminescent properties of ZnWO4: Eu3+, Dy3+ white light-emitting phosphors. J. Lumin. 172, 161 (2016)
X. Wang, Z. Fan, H.H. Yu, H.J. Zhang, J.Y. Wang, Characterization of ZnWO4 Raman crystal. Opt. Mater. Express 7, 1732 (2017)
M.T. Li, Q.H. Meng, S.Y. Li, F. Li, Q. Zhu, B.-N. Kim, J.-G. Li, Photoluminescent and photocatalytic ZnWO4 nanorods via controlled hydrothermal reaction. Ceram. Int. 45, 10746 (2019)
G.B. Kumar, K. Sivaiah, S. Buddhudu, Synthesis and characterization of ZnWO4 ceramic powder. Ceram. Int. 36, 199 (2010)
F. Dkhilalli, S.M. Borchani, M. Rasheed, R. Barille, K. Guidara, M. Megdiche, Structural, dielectric and optical properties of the zinc tungstate ZnWO4 compound. J. Mater. Sci. Mater. Electron. 29(8), 6297 (2018)
P. Yadav, S.K. Rout, E. Sinha, Correlation between optical properties and environmental parameter of ZnWO4 ceramic using complex chemical bond theory. J. Alloys Compd. 726, 1014 (2017)
P.J. Mafa, B. Ntsendwana, B.B. Mamba, A.T. Kuvarega, Visible light driven ZnMoO4/BiFeWO6/rGO z-scheme photocatalyst for the degradation of anthraquinonic dye. J. Phys. Chem. C 123, 20605 (2019)
S.H. Cao, C.F. Gao, Y. Lv, Y.J. Guo, Q. Liu, A novel BiOCl film with flowerlike hierarchical structures and its optical properties. Nanotechnology 20, 1 (2009)
Y. Liang, P. Liu, H.B. Li, G.W. Yang, ZnMoO4 micro-and nanostructures synthesized by electrochemistry-assisted laser ablation in liquids and their optical properties. Cryst. Growth Des. 12, 4487 (2012)
X.C. Song, Y.F. Zheng, E. Yang, G. Liu, Y. Zhang, H.F. Chen, Y.Y. Zhang, Photocatalytic activities of Cd-doped ZnWO4 nanorods prepared by a hydrothermal process. J. Hazard. Mater. 179, 1122 (2010)
Y.S. Shi, J.G. Shi, C. Dong, Refinement and luminescent properties of BaWO4: xSm3+ yellow phosphor by a low temperature molten salt method. Opt. Mater. 84, 396 (2018)
J. Shen, Z.X. Wang, J. Zhou, X. Liu, W. Chen, Photoluminescence properties of NUV light excited Ba(Mg1/3Nb2/3)O3: Eu3+ red phosphor with high color purity. Ceram. Int. 45, 11844 (2019)
S.Y. Cao, Q.J. Ning, C.L. Yu, C.J. Qiao, Y.S. Shi, R.C. Liu, NaSr2Nb5O15: 0.03Eu3+ phosphors via the molten salt synthesis: morphology evolution and luminescence properties. J. Alloys Compd. 691, 323 (2017)
G. Jia, D.B. Dong, C.Y. Song, L.F. Li, C.M. Huang, C.M. Zhang, Hydrothermal synthesis and luminescence properties of monodisperse BaWO4: Eu3+ submicrospheres. Mater. Lett. 120, 251 (2014)
Y.S. Shi, B. Quan, Q.J. Ning, S.Y. Cao, J.J. Shi, C. Dong, Photoluminescent properties and Judd–Ofelt analysis of novel Na0.5Sr0.25NbO3: Eu3+ red phosphor with high quantum efficiency. Mater. Res. Bull. 101, 363 (2018)
J.S. Zhong, M. Xu, D.Q. Chen, G.H. Xiao, Z.G. Ji, Novel red-emitting Sr2LaSbO6: Eu3+ phosphor with enhanced 5D0→7F4 transition for warm white light-emitting diodes. Dyes Pigm. 146, 272 (2017)
C. Zeng, Y.M. Hu, L.J. Wang, H.K. Liu, C.Y. Liu, H.W. Huang, New green-yellowish emitting fluoro-apatite compound phosphor Ba3TbK(PO4)3F: Sm3+ with high thermal stability. J. Rare Earths 35, 767 (2017)
Z.W. Gao, L. Zhao, J.Y. He, R.J. Yu, A novel green-emitting phosphor of Ce3+-activated CaGd4F14 synthesis, high efficiency, and thermal stability. Powder Technol. 331, 244 (2018)
G.Y. Dong, J.X. Zhao, M.D. Li, L. Guan, X. Li, A novel red Y2MoSiO8: Eu3+ phosphor with high thermal stability for white LEDs. Ceram. Int. 45, 2653 (2019)
M. Choi, H. Choi, J. Ahn, Y.T. Kim, Material design for Ge2Sb2Te5 phase-change material with thermal stability and lattice distortion. Sci. Mater. 170, 16 (2019)
M. Nandimath, R.F. Bhajantri, J. Naik, Effect of Rhodamine 6G dye on chromaticity co-ordinates and photoluminescence properties of TiO2/PMMA polymer nanocomposites for LED applications. J. Lumin. 207, 571 (2019)
Q. Sun, S.Y. Wang, B.L.J. Devakumar, L.L. Sun, J. Liang, X.Y. Huang, Y.C. Wu, CaYAlO4: Mn4+, Mg2+ an efficient far-red-emitting phosphor for indoor plant growth LEDs. J. Alloys Compd. 785, 1198 (2019)
Q.J. Ning, B. Quan, Y.S. Shi, Effect of alkali metal ions on the spectra of CaZn2(PO4)2: Sm3+ phosphor analyzed by J-O theory. J. Lumin. 206, 498 (2019)
G. Lifante, J.M. Mendivil, R. He, E. Cantelara, L. Ortega San Martínd, D. Sola, Transition probabilities of Er3+ ions in alumino-silicate glasses. J. Lumin. 203, 305 (2018)
J.M. de Mendívil, G. Lifante, M.C. Pujol, M. Aguiló, F. Díaz, E. Cantelar, Judd-Ofelt analysis and transition probabilities of Er3+ doped KY1-x-yGdxLuy(WO4)(2) crystals. J. Lumin. 165, 153 (2015)
C. Aleksandar, S. Stevan, M.D. Dramicanin, Luminescence intensity ratio thermometry and Judd-Ofelt analysis of TiO2: Eu3+. Opt. Mater. 85, 261 (2018)
Y.S. Shi, C. Dong, J.J. Shi, Influence of different synthesis methods on structure, morphology and luminescent properties of BiOCl: Eu3+ phosphors and J-O analysis. J. Mater. Sci. Mater. Electron. 29(1), 186 (2018)
T. Manohara, S.C. Prashantha, R. Naik, H. Nagabhushana, H.P. Nagaswarupa, K.S. Anantharaju, K.M. Girish, H.B. Premkumar, A benign approach for tailoring the photometric properties and Judd-Ofelt analysis of LaAlO3: Sm3+ nanophosphors for thermal sensor and WLED applications. Sens. Actuators B 243, 1057 (2017)
K. Nasser, V. Aseev, S. Ivanov, A. Ignatiev, N. Nikonorov, Optical spectroscopic properties and Judd-Ofelt analysis of Nd3+-doped photo-thermo-refractive glass. J. Lumin. 213, 255 (2019)
S.C. Lal, A.M. Aiswarya, K.S. Sibi, G. Subodh, Insights into the structure photoluminescence and Judd-Ofelt analysis of red emitting SrLaLiTeO6: Eu3+ phosphors. J. Alloys Compd. 788, 1300 (2019)
G. Jyothi, L.S. Kumari, K.G. Gopchandran, Site selective substitution and its influence on photoluminescence properities of Sr0.8Li0.2Ti0.8Nb0.2O3: Eu3+ phosphors. Rsc Adv. 7, 28438 (2017)
S.K. Gupta, C. Reghukumar, R.M. Kadam, Eu3+ lacal site analysis and emission characteristic of novel Nd2Zr2O7: Eu3+ phosphor insight into the effect of europium concentration on its photoluminescence properties. Rsc Adv. 6, 53614 (2016)
Acknowledgements
This work was supported by the Projects for Science and Technology of Shaanxi province (No. 2019GY-175); Program for Scientific Research of Education Department of Shaanxi province (No. 18JK0111). We would like to thank the Testing Center of the Shaanxi University of Science& Technology. We sincerely thank Jianpeng Wu, Senior Engineer of the Shaanxi University of Science& Technology, for his help with XRD analysis and so on.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ning, Q., Zhou, C. & Shi, Y. The influence of Eu3+ doping on the studies of luminescent properties and quantum efficiency of ZnWO4 phosphor. J Mater Sci: Mater Electron 31, 10499–10511 (2020). https://doi.org/10.1007/s10854-020-03598-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03598-y