Abstract
The Ca1–2x(LiCe0.5Y0.5)xBi2Nb2O9 (CBNLCY-100x, x = 0–0.07) high-temperature ceramics were prepared by the conventional solid state reaction. The LiCeY substitution at A-site led an improvement of octahedral tilt angle in the a–c plane of CBNLCY-100x ceramics, which resulted in an enhanced piezoelectric constant. CBNLCY-6 ceramic with high Curie temperature (TC = 940 °C) had a high piezoelectric coefficient (d33) of 16.3 pC/N, even after annealing at 800 °C for 2 h, the d33 value of CBNLCY-6 ceramic still remained 92.6% of its original value. Furthermore, the dc conduction mechanism of the ceramics was studied. The oxygen vacancies played an important role in dc conduction mechanism at low temperature and the main conduction mechanism at high temperature was intrinsic conduction. The resistivity of CBNLCY-100x ceramics got highest value at x = 0.01 and the resistivity of CBNLCY-1 ceramic enhanced one order of magnitude compared with undoped CaBi2Nb2O9 ceramic. These results suggest that the CBNLCY-6 ceramic is a promising candidate material for ultra-high temperature applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Calcium bismuth niobate (CaBi2Nb2O9, CBN) is one of the compounds in bismuth layer-structured ferroelectric (BLSF) family with a vast potential for applications in high temperature environment [1]. It exhibits excellent performance such as high Curie temperature (TC = 930 °C) and thermal stability [2], which make it useful over a wide temperature range. And it also possesses high resistivity and excellent fatigue-resistant properties. However, the piezoelectric activity (d33 ~ 6 pC/N) of CBN ceramics is very low due to the high coercive field (Ec) and the structural anisotropy [3], which limits its application in devices. The crystal structure of CBN ceramics is built of the interleaving (CaNb2O7)2− perovskite-like layers between with (Bi2O2)2+ sheets that are stacked along its crystallographic c-direction, where A-site ion is Ca2+ ion and B-site ion is Nb5+ ion [4, 5].
In order to improve piezoelectric property of CBN ceramics, solid solutions with other ions substitution have been considered and explored. For example, it is excepted to improve piezoelectric activity by doping with rare earth ions, alkali metal ions or combined of them at A-site and transition metal ions at B-site. Relative investigations mainly are listed in Table 1 [6,7,8,9,10,11,12,13]. It is clear that the piezoelectric properties of CBN ceramics were enhanced significantly by doping different ions. And in order to explain the electric properties of piezoelectric ceramics, the formation process and action mechanism of defect dipoles have been studied. Chen et al. [14] studied the effect of defect dipoles on dielectric relaxation and ferroelectric properties of W/Cr co-doped Bi4Ti3O12 ceramics. Long and Fan [15] reported the double-loop-like ferroelectric polarization in acceptor Aurivillius ceramic and a positive polarization contribution from defect polarization. The similar studies were previously observed in typical perovskite ferroelectrics, e.g., Cu-doped K0.5Na0.5NbO3 ceramics [16], Mg-doped K0.5Na0.5NbO3 ceramics [17] and Mn-doped Bi0.5Na0.5TiO3 ceramics [18]. In addition, the grain orientation techniques, such as template grain growth (TGG) [19] and spark plasma sintering (SPS) [20], can also improve the piezoelectricity of CBN ceramics effectively.
It is presented in Table 1 that the alkali metal ions and rare earth ions co-doping, especially lithium and cerium ions co-doping could improve the piezoelectric properties of BLSF ceramics well. But doping cerium ions (Ce3+ or Ce4+) into CBN ceramics would decrease the Curie temperature of the ceramics and limit the application in high temperature environment. While the yttrium ions (Y3+) have small ionic radii (RY3+ = 1.25 Å) and are helpful for increasing Curie temperature of the ceramics. So in this work, the Ca1–2x(LiCe0.5Y0.5)xBi2Nb2O9 (CBNLCY-100x) ceramics were prepared by solid state reaction and the crystal distortion and defect of CBNLCY-100x ceramics are studied in detail to explain the enhanced piezoelectric constant, high Curie temperature and good thermal stability of the ceramics.
2 Experiments
Ca1–2x(LiCe0.5Y0.5)xBi2Nb2O9 (CBNLCY-100x, x = 0–0.07) ceramics were prepared by solid state reactions, using CaCO3 (99%), Bi2O3 (99.999%), Nb2O5 (99.5%), Li2CO3 (99.99%), CeO2 (99.99%) and Y2O3 (99.5%) as raw materials. All powders were weighed according to the stoichiometric compositions. After ball milling with zirconia balls for 12 h, the mixed powders were dried and calcined at 900 °C for 3 h, and then the mixtures were ball-milled again for 12 h. The powders were pressed into disks of 10 mm in diameter and 0.8 mm in thickness by 8 wt% polyvinyl alcohol (PVA) under 10 MPa. After burning off PVA at 450 °C, these disks were sintered at 1080 °C–1160 °C for 3 h. All the samples were poled at 180 °C in a silicone oil bath by applying a direct current electric field of ~ 12 kV/mm for 10 min.
The crystal structures were determined using the X-ray diffraction (XRD) with Cu-Kα radiation (DX2700, Dandong, China) at room temperature. The test was conducted by 0.02° scan step and continuous scanning type in the 2θ range of 20°–60°. The powder X-ray diffraction data of CBNLCY-0 and CBNLCY-6 ceramics were collected from an automated diffractometer (Panalytical B.V. X’pert Pro) in a range of 20° to 120°. The temperature dependence of dielectric behavior of all samples were measured with an LCR analyzer (HP4980A, Agilent, USA). Their piezoelectric constant (d33) was measured using a Belincourt meter (ZJ-3A, Institute of Acoustics, Chinese Academy of Sciences, China). The dc resistivity was measured at high temperature by an electrometer (Keithley 6517B). Ferroelectric properties were determined out at room temperature using an aixACCT TF 2000 analyzer.
3 Results and discussion
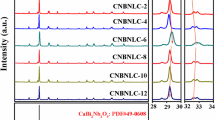
Figure 1a shows the X-ray diffraction (XRD) of CBNLCY-100x ceramics in the 2θ range of 20°–60°. The analysis for diffraction peaks indicates that the major phase of all compositions can be indexed by PDF card (PDF#49–0608, CaBi2Nb2O9). It is also found that there is less Bi2O3 (PDF 01-074-2351) existing in the matrix as the secondary phase. The formation of secondary phase Bi2O3 is due to the volatilization of bismuth element during sintering process. In the cooling process, the bismuth element reacts with oxygen to form bismuth oxide. Except Bi2O3 phase, there is only CaBi2Nb2O9 phase indicates the doping ions (Li+, Ce4+, Y3+) diffused into the lattice and formed a solid solution successfully. Figure 1b is the enlarged graph of XRD patterns in the range of 28°–30°. The highest intensity of diffraction peak is found to be (115), which is in accordance with the strongest diffraction peak (112m + 1) in BLSFs ceramics. Moreover, the (115) peak shifts to a high angle due to the substitution of smaller ions (RLi+=1.15 Å, RCe4+=1.14 Å, RY3+=1.25 Å, 12 CN) [21] for larger ions (RCa2+=1.34 Å, 12 CN) [22] in the A-site. The XRD diffraction peaks located at (200)/(020) are shown in Fig. 1c. It is clear that the (200) and (020) peaks tend to merge into a single peak with x content increasing, which demonstrates the enhancement of lattice symmetry of CBNLCY-100x ceramics [23].
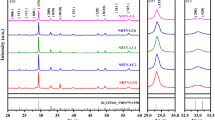
In order to confirm the lattice parameters a, b, c and characterize the crystal structural conversion of CBNLCY-100x ceramics, Rietveld refinements on powder XRD of CBNLCY-0 and CBNLCY-6 ceramics were refined using the MAUD program [24]. Figure 2a and c show the experimental and calculated XRD patterns for ceramics, the Bragg-position, and the difference between calculated and experimental intensity. The refined factors sig and Rwp, which are used to denote the quality of structural refinement, are 1.32, 8.53 for CBNLCY-0 1.16, 8.14 for CBNLCY-6 respectively, indicating a good match between the observed and calculated patterns. Figure 2b and d are the enlarged XRD patterns located at (200)/(020). It is clear that the (200) and (020) peaks tend to merge into a single peak with x content increasing and the ratio of a/b value decreases from 1.008 to 1.0048 [25]. The variation indicates that the crystal structure undergoes a phase transition from orthorhombic phase to pseudo-tetragonal phase with the introduction of dopants [26]. It implies that crystal symmetry is enhanced by the compositional variation.
The visualized crystal structures of CBNLCY-0 and CBNLCY-6 ceramics shown in Fig. 3 are obtained from refinement results. It is reported that the main crystal distortion of BLSFs ceramics occurs in NbO6 octahedron [27]. So the distortion of NbO6 octahedron is deeply studied. The difference between Nb–O bond lengths in NbO6 octahedron are decreases with the introduction of LiCeY, which implies the enhancement of octahedral symmetry. Besides, the octahedral tilt angles (α) from c axis of CBNLCY-0 and CBNLCY-6 ceramics are about 17.28° (Nb-O1-Nb bond angle: 145.45°) and 13.31° (Nb-O1-Nb bond angle: 153.39°) respectively. The decreasing tilt angle demonstrates the declining crystal distortion of CBNLCY-100x ceramics, which can also explain the variation of (200) and (020) peaks in Fig. 2b and d. In order to describe the tilt angle along a and b axis, the tilt angle in the a–c plane (αx) and in the b–c plane (αy) are listed in Table 2. It is reported that the crystal distortion related to αy do not contribute to the spontaneous polarization (Ps). The results are attributed to the glide plane perpendicular to the b axis which eliminates the ion displacement along b axis [28]. Although tilt angle (α) from c axis decreases, the tilt angle in the a–c plane increases from 1.03° to 15.82° as LiCeY content increase. The extent of octahedral rotation can be described by the rotation angle along b axis in a–b plane (β1 and β2) shown in Fig. 3. The rotation angles of CBNLCY-0 are 4.37° and 7.42°, and those of CBNLCY-6 are 4.72° and 5.82°. The change of the rotation angle is not obvious. This result indicates that the rotation of NbO6 octahedron did not make the main contribution to the ferroelectricity of CBNLCY-100x ceramics. Therefore, the enhanced lattice distortion related to αx is responsible for the larger Ps.
Figure 4 shows the polarization–electric (P–E) loops and current–electric field (I–E) curves of CBNLCY-0 and CBNLCY-6 ceramics measured at 180 °C and 1 Hz. It can be seen that the P–E loop of CBNLCY-0 ceramic exhibited cigar-shaped profile and there are no apparent ferroelectric switching peaks observed in the I–E curve during the polarization process. With the substitution of LCY dopants, the remnant polarization Pr and coercive field Ec of the ceramcis obviously vary. The Pr and Ec of CBNLCY-0 ceramic are 0.51 µC/cm2 and 3.66 kV/mm, respectively. While those of CBNLCY-6 ceramic are 1.35 µC/cm2 and 6.94 kV/mm, respectively. The enhancement of Pr is attribution to the larger Ps of CBNLCY-6 ceramic which has discussed in crystal structure analysis. Hence, the LCY doping can improve the ferroelectric properties of CBN ceramics. Figure 5 shows the fatigue characteristics of CBNLCY-6 ceramics for 105 switching cycles at 180 °C. The value of Pr do not change obviously indicating that the CBNLCY-6 ceramic have good fatigue resistance.
The electric resistivity is an important factor of BLSFs ceramics, which should be considered for piezoelectric ceramics applied in high temperature. The temperature dependence of resistivity of CBNLCY-100x ceramics are shown in Fig. 6a. The resistivity gradually declines with the increasing temperature as same as other Aurivillius type ceramics. Besides, the resistivity of CBNLCY-100x ceramics increases with enhancement of the x value and reaches the optimal value at x = 0.01 shown in Fig. 6b. The resistivity of CBNLCY-1 ceramics is 1.31 × 106 Ω·cm at 600 °C, which enhances one order of magnitude compared with undoped CBN ceramics (1.25 × 105 Ω·cm at 600 °C). In general, the oxygen vacancies make a major contribution to the electric conduction process for Aurivillius type ceramics [29,30,31]. Due to the volatilization of bismuth element during sintering, there are bismuth vacancies (\(V_{{Bi}}^{{'''}}\)) leaving at the (Bi2O2)2+ layer of CBNLCY-0 ceramics, and the oxygen vacancies (\(V_{O}^{{ \cdot \cdot }}\)) are also generated near them to maintain the charge neutrality (Eq. 1). In the cooling process, the oxygen atoms enter into (Bi2O2)2+ layer again and produce the holes (\({h^ \cdot }\)) (Eq. 2). The substitution of donor dopant Ce4+ for Ca2+ generates two electrons at A-site (Eq. 3), and neutralizes the holes (Eq. 4). Thus, the concentration of charge carriers of CBNLCY-100x ceramics decreases when x value is 0.01 mol, which makes the resistivity raise. The donor doping process can be represented by Kroger–Vink defect-chemical equation [32]:
a Resistivity of CBNLCY-100x ceramics as a function of measuring temperature; b resistivity as a function of x for CBNLCY-100x ceramics at 400, 500, 600 and 700 °C; c–i Arrhenius relationship between dc resistivity and temperature for the CBNLCY-100x ceramics; j activation energy (Ea) obtained from the Arrhenius plots and the transition temperature Tchange corresponding to the changes of Ea
With the further increase of x value, the resistivity of CBNLCY-100x ceramics decreases. It is reported that the lanthanide metals Ce4+ also have chance to entered into B-site of the perovskite structure to replace the Nb5+ because of their similar ionic radii (RCe4+ = 0.87 Å, RNb5+ = 0.64 Å, 6 CN) [33]. When the low valence Ce4+ ions enter into B-site to replace Nb5+ ions, one oxygen vacancy (\(V_{O}^{{ \cdot \cdot }}\)) will be generated at B-site of perovskite layer [34]. The Kroger–Vink defect-chemical equation of acceptor doping process are shown in Eq. (5). Hence, the concentration of charge carriers increases and makes the resistivity reduce.
Furthermore, the temperature-dependent conduction process follows the Arrhenius equation [35]:
where ρ0 is the pre-exponential factor, Ea is the activation energy of the charge carriers, kB is the Boltzmann’s constant and T is the absolute temperature. It can be seen that all curves can be divided into two temperature regions. The value of Ea at low temperature is close to 1 eV, which is attribution to the motion of oxygen vacancy [36]. And at high temperature, the values of Ea are near half of the band gap (1.7 eV), proving intrinsic conduction is the main conduction mechanism [37]. When x value is 0.01 mol, the Ea of CBNLCY-100x ceramics has a slight decrease at low temperature but an increase at high temperature, which is ascribed to the decreasing concentration of oxygen vacancies. While with the x content further increasing, it can be observed that the activation energy increases at low temperature while declines at high temperature, and the values of Ea in two temperature regions are both tend to 1 eV. It demonstrates that the Ce4+ ions enter into B-sites and generate more oxygen vacancies. The schematic in Fig. 7 explains the defect formation process of the CBNLCY-100x ceramics in detail. This result is consistent with the analysis of the variation of resistivity.
Figure 8a shows the temperature dependence of dielectric constant (ɛr) of the CBNLCY-100x ceramics measured at 1 MHz. The dielectric peaks around 940 °C are the phase transition points between ferroelectric and paraelectric phase. The Curie temperature (TC) of CBNLCY-100x ceramics increases firstly with x content increasing while decreases with the further doping. In order to further elucidate the effect of LiCeY co-substitution on TC, the tolerance factor (t) of the perovskites is invoked [12]. The formula of tolerance factor (t) is given by Eq. (7):
where RA, RB, and RO are the radii of A, B-site and oxygen atoms, respectively. In a certain range, the lower t value often causes higher TC. Compared with Ca2+ ion (RCa2+ = 1.34 Å, 12 CN), the Li+, Ce4+ and Y3+ ions (RLi+ = 1.15 Å, RCe4+ = 1.14 Å, RY3+ = 1.25 Å, 12 CN) have smaller radii which lead a lower t value. So the TC presents an uptrend with x content increasing and reaches the highest TC value (949 °C) at x = 0.02. Meanwhile, excessive LiCeY makes the crystal symmetry enhanced, resulting in a decrease of TC when x ≥ 0.02. It is clear that the dielectric loss (tanδ) of CBNLCY-100x ceramics below 600 °C is flat, but increase rapidly near Curie points because of the movement of domain walls. Figure 8d shows the variation of tanδ with the x value increasing at 600 °C. It is found that the CBNLCY-100x ceramics obtain the lowest dielectric loss at x = 0.01. With the addition of less LiCeY (x = 0.01) into A-site, the oxygen vacancies generated by the volatilization of bismuth are neutralized by high valence Ce4+ ions, which result in the lower dielectric loss [38].
Piezoelectric coefficient (d33) and its thermal stability of CBNLCY-100x ceramics are important parameters for high Curie temperature piezoceramics. Figure 9a shows the piezoelectric constant d33 of CBNLCY-100x ceramics at room temperature. As shown, the d33 value increases and then stays nearly constant with the increase of the LiCeY content. The optimal piezoelectricity is obtained at x = 0.06 with a d33 value of 16.3 pC/N, which is much larger than that of undoped CBN ceramics (d33 = 6.6 pC/N). The crystal distortion can explain the great enhancement of d33 value. It has been studied that the octahedral tilt angle in the a–c plane (αx) is the main contribution to the ferroelectricity of CBNLCY-100x ceramics [28]. Compared with CBNLCY-0 ceramic, CBNLCY-6 ceramic has larger αx value, and CBNLCY-6 ceramic also has larger remnant polarization Pr shown in Fig. 4. Therefore, the CBNLCY-6 ceramic obtained the high piezoelectric constant. Figure 9b shows the thermal stability of CBNLCY-100x ceramics. One could see that all the samples have stable piezoelectric properties. The d33 value drops slightly when the annealing temperature rises to 400 °C, which is attribution to the movement of non-180° domain walls [21, 39]. When the annealing temperature is close to Curie temperature, the d33 value decreases rapidly, indicating the phase transition from ferroelectric phase to paraelectric phase. Figure 9c gives the relative d33 of CBNLCY-6 ceramic after annealing at various temperatures. It is observed that the relative d33 value of CBNLCY-6 ceramics still remains 92.6% of its original value, which indicates the CBNLCY-6 ceramic has good resistance to thermal aging.
4 Conclusions
The (LiCeY) co-doped Ca1–2x(LiCe0.5Y0.5)xBi2Nb2O9 (CBNLCY-100x, x = 0–0.07) ceramics were prepared by the conventional solid-state reaction. The substitution of (LiCeY) at A-site led an improvement of the octahedral tilt angle in the a–c plane of CBNLCY-100x ceramics, which resulted in an enhanced piezoelectric property. CBNLCY-6 ceramic with high Curie temperature (TC = 940 °C) had an enhanced piezoelectric coefficient (d33) of 16.3 pC/N. Even after annealing at 800 °C for 2 h, the d33 value of CBNLCY-6 ceramic still remained 92.6% of its original value. Furthermore, detailed investigations about the resistivity of CBNLCY-100x ceramics showed that the oxygen vacancies played an important role in dc conduction mechanism at low temperature and the main conduction mechanism at high temperature was intrinsic conduction. The high valence state ions Ce4+ entered into A-site of perovskite to reduce the concentration of oxygen vacancies, finally enhanced the resistivity of CBNLCY-100x ceramics and decreased the dielectric loss. But with the further modification, the Ce4+ might enter into B-site as an acceptor and generated more oxygen vacancies. Ultimately, CBNLCY-1 ceramic got the highest resistivity (1.31 × 106 Ω cm) and lowest tanδ (~ 0.03) at 600 °C.
References
S.M. Blake, M.J. Falconer, M. Mccreedy, P. Lightfoot, J. Mater. Chem. 7, 1609 (1997)
H. Chen, B. Shen, J. Xu, L. Kong, J. Zhai, J. Am. Ceram. Soc. 95, 3514 (2012)
C.M. Wang, S. Zhang, J.F. Wang, M.L. Zhao, C.L. Wang, Mater. Chem. Phys. 118, 21 (2009)
E.C. Subbarao, J. Phys. Chem. Solids 23, 665 (1962)
E.C. Subbarao, J. Am. Ceram. Soc. 45, 166 (1962)
X. Zhang, H. Yan, M.J. Reece, J. Am. Ceram. Soc. 91, 2928 (2008)
X.X. Tian, S.B. Qu, H.L. Du, Y. Li, Z. Xu, Chin. Phys. B 21, 037701 (2012)
Z. Peng, Q. Chen, Y. Wang, D. Xin, D. Xian, J. Zhu, Mater. Lett. 107, 14 (2013)
C.M. Wang, J.F. Wang, S. Zhang, T.R. Shrout, Phys. Status Solidi RRL 3, 49 (2009)
Y. Wang, J. Wu, Z. Peng, Q. Chen, D. Xin, D. Xiao, J. Zhu, Appl. Phys. A 119, 337 (2015)
J. Xie, J. Zhong, C. Wu, Y. Shi, D. Wang, G. Liu, D. Liang, B. Wang, J. Zhu, Q. Chen, J. Am. Ceram. Soc. 00, 1 (2018)
C. Long, H. Fan, M. Li, P. Ren, Y. Cai, Cryst. Eng. Commun. 15, 10212 (2013)
G. Liu, J. Yuan, R. Nie, L. Jiang, Z. Tan, J. Zhu, Q. Chen, J. Alloy. Compd. 697, 380 (2016)
Y. Chen, S. Xie, H. Wang, Q. Chen, Q. Wang, J. Zhu, Z. Guan, J. Alloy. Compd. 696, 746 (2017)
C. Long, H. Fan, M. Li, Appl. Phys. Lett. 103, 192908 (2013)
S.M. Ke, H.T. Huang, H.Q. Fan, H.K. Lee, L.M. Zhou, Y.W. Mai, Appl. Phys. Lett. 101, 082901 (2012)
T. Li, H. Fan, C. Long, G. Dong, S. Sun, J. Alloy. Compd. 609, 60 (2014)
Y. Guo, H. Fan, C. Long, J. Shi, L. Yang, S. Lei, J. Alloy. Compd. 610, 189 (2014)
H. Chen, J. Zhai, J. Electron. Mater. 41, 2238 (2012)
J. Liu, Z. Shen, M. Nygren, Y. Kan, P. Wang, J. Eur. Ceram. Soc. 26, 3233 (2006)
C. Long, H. Fan, M. Li, Dalton Trans. 42, 3561 (2013)
R.D. Shannon, Acta Crystallogr. Sect. A Found. Adv. 32, 751 (1976)
Y. Wu, J. Chen, J. Yuan, J. Xing, Z. Tan, L. Jiang, Q. Chen, J. Zhu, J. Appl. Phys. 120, 194103 (2016)
L. Xie, J. Xing, Z. Tan, L. Jiang, Q. Chen, J. Wu, W. Zhang, D. Xiao, J. Zhu, J. Alloy. Compd. 758, 14 (2018)
J. Yuan, J. Chen, S. Bao, Q. Chen, D. Xiao, J. Zhu, J. Mater. Sci. Mater. Electron. 29, 21051 (2018)
Z. Peng, X. Xing, X. Zeng, Y. Xiang, F. Cao, B. Wu, Mater. Res. Bull. 97, 393 (2018)
Y. Noguchi, M. Miyayama, T. Kudo, Phys. Rev. B 63, 214102 (2012)
Z.G. Yi, Y. Wang, Y.X. Li, Q.R. Yin, J. Appl. Phys. 99, 114101 (2006)
H. Chen, X. Guo, Z. Cui, J. Zhai, Phys. Status Solidi A 210, 1121 (2013)
J. Chen, J. Yuan, S. Bao, Y. Wu, G. Liu, Q. Chen, D. Xiao, J. Zhu, Ceram. Int. 43, 5002 (2017)
C. Long, H. Fan, Y. Wu, Y. Li, J. Appl. Phys. 116, 074111 (2014)
Z. Peng, Y. Chen, Q. Chen, N. Li, X. Zhao, C. Kou, D. Xiao, J. Zhu, J. Alloy. Compd. 590, 210 (2014)
Y. Tsur, T.D. Dunbar, C.A. Randall, J. Electroceram. 7, 25 (2001)
Z. Peng, Q. Chen, D. Liu, Y. Wang, D. Xiao, J. Zhu, Curr. Appl. Phys. 13, 1183 (2013)
Z. Zhou, X. Dong, H. Chen, H. Yan, J. Am. Ceram. Soc. 89, 1756 (2006)
Z. Zhou, X. Dong, H. Yan, H. Chen, C. Mao, J. Appl. Phys. 100, 044112 (2016)
Z. Peng, Q. Chen, Y. Chen, D. Xiao, J. Zhu, Mater. Res. Bull. 59, 125 (2014)
Z. Peng, X. Xing, Y. Xiang, F. Cao, B. Wu, J. Alloy. Compd. 728, 797 (2017)
Z. Peng, D. Yan, Q. Chen, D. Xin, D. Liu, D. Xiao, J. Zhu, Curr. Appl. Phys. 14, 1861 (2014)
Acknowledgements
This work was supported by the Key Program of National Natural Science Foundation of China (No. 51332003) and Sichuan Science and Technology Program (2018G20140).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, J., Nie, R., Li, W. et al. Impact of crystal structure and defect on the electric properties in (LiCeY)-doped CaBi2Nb2O9-based high-temperature piezoceramics. J Mater Sci: Mater Electron 30, 5240–5248 (2019). https://doi.org/10.1007/s10854-019-00823-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-00823-1