Abstract
Aurivillius phase [Ca0.6(Na0.5Bi0.5)0.4]1−x(LiCe)x/2Bi2Nb2O9 (CNBNLC-100x, x = 0, 0.02, 0.04, 0.06, 0.08, 0.10, 0.12) ceramics were prepared by the conventional solid state method. The LiCe substitution induced structural distortion and orthorhombic distortion of CNBNLC-100x ceramics which influenced piezoelectric activities and Curie temperature, respectively. The results implied that the more structural distortion of lattice leads to higher piezoelectricity. CNBNLC-8 ceramics with ultrahigh TC of 904 °C had a high piezoelectric activity of 17.6 pC/N, even after annealing at 600 °C for 2 h, the d33 value of CNBNLC-8 ceramics still retained 62.5% of its original value. Ce4+ as donor dopant, the dc resistivity of CNBN ceramics increased with the substitution of Ce4+ for Ca2+. At high temperature region, the extrinsic conductivity changes of the ceramics from p-type to n-type with the increase of Ce4+ doping amount. Further investigation demonstrated that the ac conductive process of ceramics referred to three different mechanisms. The main conduction mechanisms were considered as first-order ionization of oxygen vacancies, second- order ionization of oxygen vacancies and intrinsic dc conductivity at three temperature regions, respectively. All these good electrical properties provided the LiCe doped CNBN ceramics with a large potentiality for high-temperature piezoelectric applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aurivillius-type bismuth layer-structured ferroelectrics (BLSFs) have attracted lots of attention in recent years for their potential applications in ferroelectric/piezoelectric devices used in the high-temperature and high-frequency environment [1,2,3], since they have many unique properties, such as high Curie temperature, low dissipation factor and excellent fatigue endurance property [4, 5]. But the major defect of BLSFs ceramics is the low piezoelectric activity which limits their applications. A general formula of BLSFs is (Bi2O2)2+(Am−1BmO3m+1)2− where A is mono-, di-, tri-valent ion (or a combination of them) allowing dodecahedral coordination, e.g., Ca2+, Sr2+, Ba2+, Pb2+, Bi3+, etc., B is a combination of transition metal cations suited for octahedral coordination, like Nb5+, Ta5+, Ti4+, W6+, Zr4+, etc., m is the number of octahedral layers in the perovskite slab and its value varies from 1 to 6.
Both calcium bismuth niobate (CaBi2Nb2O9, CBN) and sodium bismuth niobate (Na0.5Bi2.5Nb2O9, NBN) are BLSF members with m = 2. CBN has a high Curie temperature (TC=940 °C) but a low piezoelectric coefficient (d33 = 5.8 pC/N) [6, 7], while NBN possesses a lower Curie temperature (TC=790 °C) but a higher piezoelectric coefficient (d33 = 17 pC/N) [8]. Hence, if (Na0.5Bi0.5)2+ can enter A site to substitute for Ca2+, the formed solid solution Ca1−x(Na0.5Bi0.5)xBi2Nb2O9 (CNBN) may be expected to keep a high Curie temperature (TC) as well as gain an ideal piezoelectric constant (d33), and its properties could be adjusted by the change of (Na0.5Bi0.5) content. An experiment shows that d33 increases but TC decreases with the increase of (Na0.5Bi0.5) content from 0 to 1 carried out by X. Tian [9]. Especially, a specific composition of Ca0.6(Na0.5Bi0.5)0.4Bi2Nb2O9 has a desirable TC and moderate d33. In addition, to improve piezoelectric activity, A-site or B-site cations modified BLSFs have been investigated. Especially, co-substitution of alkali metal cations (Li+, K+, Na+, etc.) and lanthanon cations (La3+, Ce4+, Nd3+, Pr3+, etc.) at the A-site are proved to be more effective [10,11,12,13,14,15,16,17,18,19].
In this work, LiCe-doped CNBN ceramics were prepared by the mixed oxide routes, which were aiming at attaining a high resistivity and a large piezoelectric activity as well as addressing the conduction mechanisms within BLSFs. The correlation between structural distortion and piezoelectric properties was also studied in detail to understand the effect of Li+ and Ce4+ co-substitution.
2 Experimental
Aurivillius type [Ca0.6(Na0.5Bi0.5)0.4]1−x(LiCe)x/2Bi2Nb2O9 (CNBNLC-100x, x = 0, 0.02, 0.04, 0.06, 0.08, 0.10, 0.12) ceramics were prepared by a conventional solid-state reaction route. Bi2O3 (99%), CaCO3 (99%), Na2CO3 (99.8%), Nb2O5 (99.5%), Li2CO3 (99.99%) and CeO2 (99.99%) were used as starting raw materials. All powders were weighed according to the stoichiometric compositions and then mixed by ball milling in ethanol for 12 h. The mixtures were dried and then calcined at 850 °C for 3 h in an alumina crucible. The calcined powders were milled again in the same condition and granulated with polyvinyl alcohol (PVA) as a binder. The powders were finally pressed into disks of 10 mm in diameter and 1 mm in thickness by a steel die at a uniaxial pressure of about 10 MPa. After burning out the binder at 850 °C, the samples were sintered at 1080–1120 °C for 3 h in air. For the electrical measurement, silver electrodes were painted on both surfaces of the disks at 700 °C for 10 min. The samples were poled at 180 °C in a silicone oil bath for 20 min under dc electric field of 10–12 kV/mm and then cooled to room temperature.
The crystal structures of CNBNLC-100x ceramics were determined by X-ray diffractometer (DX2700, Dandong, China) employing Cu-Kα radiation (λ = 1.5418 Å) at a scan rate of 0.02°/s. The natural surfaces of sintered ceramics were observed by the scanning electron microscope (S-3400N, Hitachi, Japan). The temperature dependence of dielectric constant and dielectric loss of the ceramics were measured using a programmable furnace with an LCR analyzer (HP 4980A, Agilent, US). The piezoelectric constant d33 were determined using a Berlincourt-type d33 meter (ZJ-3A, Institute of Acoustics, Chinese Academy of Sciences, China). High-temperature electrical resistivity was tested by a resistance meter (Partul HRMS-900) at 10 kV from 200 to 710 °C. Besides, the thermal depoling experiments were conducted by annealing the poled samples for 2 h at each selected temperature, and measuring the d33 value after they were cooled to room temperature.
3 Results and discussion
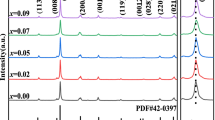
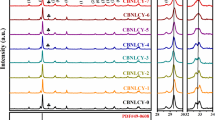
Figure 1 shows the XRD patterns of the CNBNLC-100x ceramics in the 2θ range of 20°–70°, which do not find obvious secondary phase. It indicates that the doping ions successfully diffused into the perovskite-like structure and formed a solid solution. As presented in Fig. 1(a) and (b), the diffraction peaks of CNBNLC-100x ceramics shift slightly to higher angles with the value of x varying from 0 to 0.06, which should be ascribed to the lattice shrinkage caused by the substitution of smaller Li+ and Ce4+ (Li+~ 0.76 Å, Ce4+~ 0.87 Å) [18] for larger Ca2+, Na+ and Bi3+ ions (Ca2+~ 1.34 Å, Na+~ 1.02 Å, Bi3+~ 1.36 Å) [20]. With LiCe content further increasing, the diffraction peaks shift gradually to the lower angles. Because the valent state of Ce cation changes from 4 + to 3 + and the larger Ce3+ diffused into the lattice. The shift of diffraction peaks implies that the LiCe doping leads to lattice distortion. In order to characterize the structures of CNBNLC-100x ceramics, XRD Rietveld refinements were carried out by using MAUD program [21]. Figure 2 shows the refined factors and lattice parameters of CNBNLC-100x ceramics. The refined factors indicate a good match between the observed and calculated patterns. Meanwhile, the atomic positions are listed in Table 1. According to the refinement result, perovskite unite, Bi2O2 layers structures and bond distances of CNBNLC-100x (x = 0, 0.04, 0.08, 0.12) ceramics are shown in Fig. 3. For CNBNLC-100x ceramics, the tilt angle along c axis of NbO6 octahedron has a growing trend with x increasing from 0 to 0.08 (11° for CNBNLC-0, 13° for CNBNLC-4, 20° for CNBNLC-8) owing to the substitution of smaller ions (Li+, Ce4+) for large ions (Ca2+, Na+, Bi3+) in A-site. Then the tilt angle decreases with x further increasing to 0.12 (9° for CNBNLC-12) due to the variety of Ce valence [22]. In order to characterize the distortion of crystal structure, the sum of the squared deviations of bond lengths from the average is defined as structural distortions Δ [23]:
where Z is the coordination number, R is the individual bond length, and \(\bar {R}\) is the average bond length. The structural distortion of CNBNLC-100x (x = 0, 0.04, 0.08, 0.12) ceramics are shown in Fig. 4. The distortion of the perovskite unit changes gradually but that of the Bi2O2 layer increases first and then decreases with the enhancement of LiCe contents. Both NbO6 octahedron and Bi2O2 layer of CNBNLC-8 ceramics have the largest structural distortion.
Figure 5 shows SEM images of the natural surface and the average grain sizes of CNBNLC-100x ceramics sintered at 1100 °C. The micrographs with the presence of well-packed and pore-free microstructure indicate that dense samples were achieved by this ceramic process [24]. Further, the average grain size values of CNBNLC-100x ceramics are shown in Fig. 5h. It can be clearly seen that the grain size of CNBNLC-8 ceramic is significantly bigger compared with other compositions. Its grain size is found to be 12 µm in length scale and 3 µm in thickness scale. The corresponding aspect ratio (l/t) is thus determined as 4. However, with increasing contents of LiCe, the aspect ratio (l/t) of samples decline significantly, implying that excess LiCe dopants delays the diffusion mechanisms of a-b plane and decreases the anisotropic feature of microstructure [25]. The element analysis of CNBNLC-8 ceramics is obtained by EDS shown in Fig. 5j. All elements are found except Li element. The Li atoms cannot be detected due to their rather light atomic mass. The nominal Ca/Ce ratio in CNBNLC-8 ceramics 13.8 while the measured Ca/Ce ratio is 14.45, which slightly deviates from the stoichiometry.
SEM images of natural surface and the average grain sizes of CNBNLC-100x ceramics sintered at 1100 °C: a CNBNLC-0; b CNBNLC-2; c CNBNLC-4; d CNBNLC-6; e CNBNLC-8; f CNBNLC-10; g CNBNLC-12; h the average grain sizes of CNBNLC-100x ceramics; i The section of CNBNLC-8 ceramics; j the EDS analysis of CNBNLC-8 ceramics
Figure 6a and b presents the relative permittivity εr and dielectric loss tanδ measured at 1 MHz as a function of temperature for the CNBNLC-100x ceramics. The dielectric peak of ferro-paraelectric phase transition slightly shifts to a higher temperature at x = 0.04 (TC=905 °C) and then shift to lower temperature with x increasing shown in inset Fig. 6(a). It is known that the value of Curie temperature TC is correlated with the pseudo-perovskite distortion. In consequence, the orthorhombicity of the ceramics defined as 2(a − b)/(a + b), where the a and b are the lattice parameters [26]. The relationship between TC and orthorhombicity is shown in Fig. 6c and both of them exhibit the same variation trend. In Fig. 6b, it is clear that the loss of CNBNLC-100x ceramics rises with the increase of LiCe contents at high temperature. This phenomenon indicates that the massive LiCe dopants could reduce resistivity.
The piezoelectric coefficient d33 values of CNBNLC-100x ceramics are shown in Fig. 7a. The piezoelectric activity of the ceramics was improved by increasing LiCe content but decreased by excess LiCe. It can be clearly seen that d33 value and structural distortion of CNBNLC-100x ceramics have the similar trend. The d33 of CNBNLC-100x ceramics get the maximum value of 17.6 pC/N at x = 0.08, which is consistent with structural distortion. That is to say, the introducing of LiCe dopants induces structural distortion and leads to larger piezoelectric activity. In addition, it was reported that the domain motion is much easier in coarse grains than that in fine grains [25] which means the increasing grain size will help improve piezoelectric properties. It can be seen in Fig. 5h that CNBNLC-8 ceramics with the largest grain size gain the highest d33 value. Figure 7b shows the thermal stability of relative d33 of CNBNLC-100x ceramics and the inset Fig. 7b presents thermal stability of the piezoelectric coefficient d33 of CNBNLC-100x ceramics. For CNBNLC-0 and CNBNLC-2 ceramics, the d33 value changes slightly with the annealing temperature raising. The degradation of d33 at low temperature is due to the thermally activated reverses switching of the non-180° ferroelectric domains. As seen clearly from these d33-T curves, undoped CNBNLC-0 ceramics and the lowly-doped CNBNLC-2 ceramics have better thermal stability than those of highly-doped ones. The phenomenon could be attributed to the significant depoling of the space polarization (such as point defect, etc.) within doped BLSFs ceramics generated by the thermal activation [27, 28]. However, even after annealing at 600 °C for 2 h, the d33 value of CNBNLC-8 ceramics could retain ~ 11 pC/N (62.5% of its original values), proving that the material is suitable for working at high temperatures.
Besides piezoelectric activity, the electric conductivity is another key factor which should be considered for piezoceramics applied in high temperature. Figure 8a shows the resistivity of the CNBNLC-100x ceramics as a function of temperature from 200 to 500 °C. As the same as other Aurivillius type ceramics, the electrical conduction mechanism of CNBN ceramics is also a mixed p-type electrical conduction related to the volatilization of bismuth element during sintering. However, in the cooling process, oxygen atoms in the atmosphere tend to enter into the crystal lattices of ceramics again, generating the holes (h˙) by the compensating mechanism of unbalanced valence. When the high-valent donor Ce4+ substitutes for Ca2+ in CNBNLC-100x ceramics, there will be two redundant electrons created at A-site for per substitution. Thus the oxygen vacancies resulted from the bismuth volatilization can be eliminated by the neutralization of those electrons with the help of holes. Finally, the concentration of charge carriers in CNBNLC-100x ceramics decreases. Based on this conduction mechanism, the resistivity of CNBN ceramics at high temperature can be improved by doping Ce4+. The donor doping can be described by the following Kroger-Vink defect-chemical equations:
The resistivity at different temperatures as a function of x for CNBNLC-100x ceramics is shown in Fig. 8(b), and the dc resistivity at 500 °C and 600 °C of all the sample have the same order of magnitude as shown in Table 2. The CNBNLC-2 ceramic reaches the highest resistivity of 4.82 × 106 Ω cm at 500 °C and 5.33 × 105 Ω cm at 600 °C among all the samples. The improvement in resistivity of CNBNLC-100x ceramics is attributed to the decreased concentration of charge carriers including holes and oxygen vacancies by doping LiCe. The CNBNLC-2 sample reaches the highest resistivity indicates its concentration of charge carriers has been decreased to the minimum value by doping LiCe with an appropriate content [29]. However, if the doping content of LiCe continually increases, the ceramics will be charged to an n-type semiconductor by the overmuch electrons created by the LiCe substitution for Ca2+, thus the resistivity exhibits a continuous downtrend as increasing from 0.04 to 0.12, which is described in Fig. 8b.
Furthermore, the behavior of temperature dependent conduction follows the Arrhenius relationship:
where σ0 is the pre-exponential factor, Ea is the activation energy of the charge carriers, kB is the Boltzmann’s constant and T is the absolute temperature. The Arrhenius plots of σ versus 1/T of the CNBNLC-100x ceramics are shown in Fig. 9. All conduction behavior can be described as two different temperature regions except CNBNLC-0 ceramics. According to the linearly fitting for the curve, the activation energy Ea is estimated to be from 1.21 to 1.36 eV at high temperature as shown in Fig. 9. The similar values of Ea, which seems to be independent of the LiCe dopant amount, which can further prove that the intrinsic conduction is predominant for CNBNLC-100x ceramics at high temperature. It has been reported that the intrinsic electronic conductivity activation energy is equal to half of the band gap Eg [30]. At low temperature, the activation energy is found to be 0.76–0.98 eV. It is close to the value of oxygen vacancies (~ 1 eV) involved in the extrinsic electrical conduction [31]. As a consequence, the conductivity mechanism at low temperature is considered as an ionic-electronic mixed conduction. In Fig. 9, it also shows that the transition temperature from extrinsic conduction to intrinsic conduction increases with increasing LiCe donor dopants. Because of the contribution from the electrons created by excessive Ce4+ amount, the low-temperature range dominated by the extrinsic conduction becomes wider [32].
The temperature dependence of the σac can be predicted by the dielectric properties of CNBNLC ceramics [33]:
where the σac is the total conductivity, where ω is angular frequency, ɛ0 is the permittivity of vacuum, ɛr is the relative permittivity, tanδ is the dielectric loss. Figure 10 shows the σac of CNBNLC-8 ceramics as a function of temperature at different frequencies. Obviously, the ac conduction behavior of the ceramics can be divided into three temperature regions at different frequencies. According to the slope of three fitting linear at the three regions, the values of activation energy Ea are calculated to be 0.1 eV, 0.6 eV, and 1.66 eV respectively. In region-I, the σac values are weakly temperature dependent and with the increase of test frequency, the ac conductivity increases by the order of magnitude. The obtained value of activation energy (~ 0.1 eV) indicates that the free conduction electrons resulted from the first-order ionization of oxygen vacancies. In region-II, the σac values increase rapidly above 460 °C at low frequency and all curves tend to merge. The observed Ea value (~ 0.6 eV) is very close to 0.7 eV which is the half of second-order ionization energy of oxygen vacancies. With further increasing temperatures (region-III, T > 690 °C), however, the Ea value (~ 1.66 eV) is equal to half the energy of the band gap, indicating that the conduction mechanism is referred to the intrinsic dc conductivity [34].
Figure 11 shows the ac conductivity of CNBNLC-8 ceramic as a function of measured frequency between 450 and 650 °C. It is found that the ac conductivity can be separated into two regions. The σac values remain constant approximately at low frequency region which implies that the dc conductivity dominates this process; Meanwhile, at high frequency region, the conductivity starts to increase non-linearly with increasing frequency. The frequency dependence of σac can be described by Jonscher’s power law:
where the σ (ω) is the total conductivity, the σdc is the dc conductivity, the coefficient A and exponent n (0 < n < 1) are constants depend on temperature and material intrinsic property. After fitting curves at varies temperature, exponent n shows a minimum around 550 °C which is consistent with the temperature range of dielectric loss anomaly [35, 36].
4 Conclusions
In summary, the LiCe-doping CNBN-based ceramics have a promising application for piezoelectric sensors under high temperature. CNBNLC-8 ceramic has both of high piezoelectric coefficient (17.6 pC/N) and Curie temperature (904 °C) which are owing to the structural distortion and orthorhombic distortion of the lattice. Even annealing at 600 °C for 2 h, the d33 value of CNBNLC-8 ceramics retained 62.5% of its original value. All compositions of CNBNLC-100x ceramics have high resistivity. The dc conductivity decreases with the amount of donor dopant increasing. With further increase of LiCe dopant, the dc conduction mechanism of the ceramics changes from p-type to n-type. The ac conduction mechanism of CNBNLC-8 ceramics below 690 °C is attributed to first-order ionization and second-order ionization oxygen vacancies and the main mechanism above Curie temperature is intrinsic dc conductivity.
References
D. Damjanovic, Curr. Opin. Solid State Mater. Sci. 3, 469 (1998)
S. Zhang, F. Yu, J. Am. Ceram. Soc. 94, 3153 (2011)
A.P. Barranco, Y.G. Abreu, J. Adv. Dielectr. 3, 1330003 (2013)
M. Villegas, T. Jardiel, A.C. Caballero, J.F. Fernández, J. Electroceram. 13, 543 (2004)
A. Moure, L. Castro, Pardo, Acta Mater. 52, 945 (2004)
H. Yan, H. Zhang, R. Ubic, M.J. Reece, J. Liu, Z. Shen, Z. Zhang, Adv. Mater. 17, 1261 (2005)
H. Chen, J. Zhai, J. Electron. Mater. 41, 2238 (2012)
R. Aoyagi, H. Takeda, S. Okamura, T. Shiosaki, Mater. Res. Bull. 38, 25 (2003)
X. Tian, S. Qu, B. Wang, Z. Xu, Mater. Res. Innov. 19, 171 (2015)
C.M. Wang, J.F. Wang, S. Zhang, T.R. Shrout, J. Appl. Phys. 105, 094110 (2009)
H. Yan, Z. Zhang. W. Zhu, L. He, Y. Yu, C. Li, J. Zhou, Mater. Res. Bull. 39, 1237 (2004)
C.M. Wang, J.F. Wang, Appl. Phys. Lett. 89, 202905 (2006)
X. Zhang, H. Yan, M.J. Reece, J. Am. Ceram. Soc. 91, 2928 (2008)
Z. Peng, F. Huang, Q. Chen, S. Bao, Y. Wang, D. Xiao, J. Zhu, Ferroelectrics 447, 69 (2013)
P. Xiao, Y. Guo, M. Tian, Q. Zheng, N. Jiang, X. Wu, Z. Xia, D. Lin, Dalton Trans. 44, 17366 (2015)
Z. Peng, Q. Chen, D. Liu, Y. Wang, D. Xiao, J. Zhu, Curr. Appl. Phys. 13, 1183 (2013)
Z.G. Gai, J.F. Wang, M.L. Zhao, W.B. Sun, S.Q. Sun, B.Q. Ming, P. Qi, L.M. Zheng, J. Du, C.M. Wang, S. Zhang, T.R. Shrout, Scr. Mater. 59, 115 (2008)
C. Long, H. Fan, M. Li, Dalton Trans. 42, 3561 (2013)
D. Xin, Z. Peng, F. Huang, Q. Chen, J. Wu, Y. Wang, X. Yue, D. Xiao, J. Zhu, J. Mater. Sci. 27, 913 (2016)
D.Y. Suárez, I.M. Reaney, W.E. Lee, J. Mater. Res. 16, 3139 (2001)
L. Jiang, Y. Li, L. Xie, J. Wu, Q. Chen, D. Xiao, J. Zhu, J. Mater. Sci. 28, 17761 (2017)
Y. Noguchi, M. Miyayama, T. Kudo, Phys. Rev. B 63, 214102 (2001)
Y. Shimakawa, Y. Kubo, Y. Tauchi, T. Kamiyama, H. Asano, F. Izumi, Appl. Phys. Lett. 77, 2749 (2000)
H. Yan, C. Li, J. Zhou, W. Zhu, L. He, Y. Song, Y. Yu, Mater. Sci. Eng. B 88, 62 (2002)
H. Chen, B. Shen, J. Xu, L. Kong, J. Zhai, J. Am. Ceram. Soc. 95, 3514 (2012)
B.J. Kennedy, Q. Zhou, Y. Ismunandar, K. Kubotac, Kato, J. Solid State Chem. 181, 1377 (2008)
T. Zeng, H. Yan, M.J. Reece, J. Appl. Phys. 108, 096101 (2010)
H. Yan, H. Zhang, M.J. Reece, X. Dong, Appl. Phys. Lett. 87, 082911 (2005)
T. Jardiel, A.C. Caballero, M. Villegas, J. Eur. Ceram. Soc. 27, 4115 (2007)
Z. Zhou, X. Dong, H. Yan, H. Chen, C. Mao, J. Appl. Phys. 100, 044112 (2006)
Y. Chen, Z. Peng, Q. Wang, J. Zhu, J. Alloys Compd. 612, 120 (2014)
Z. Zhou, X. Dong, H. Chen, J. Am. Ceram. Soc. 89, 1756 (2006)
Z. Peng, Q. Chen, J. Wu, D. Liu, D. Xiao, J. Zhu, J. Alloys Compd. 541, 310 (2012)
J.S. Kim, M.S. Jang, I.W. Kim, K.S. Lee, J. Electroceram. 17, 129 (2006)
T. Mondal, S. Das, T. Badapanda, T.P. Sinha, P.M. Sarun, Phys. B 508, 124 (2017)
H.S. Shulman, D. Damjanovic, N. Setter, J. Am. Ceram. Soc. 83, 528 (2000)
Acknowledgements
This work was supported by National Natural Science Foundation of China (51332003) and Sichuan Science and Technology Program (2018G20140).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yuan, J., Chen, J., Bao, S. et al. Effect of doping on the structure, piezoelectric and electrical properties of Aurivillius type Ca0.6(Na0.5Bi0.5)0.4Bi2Nb2O9 ceramics. J Mater Sci: Mater Electron 29, 21051–21060 (2018). https://doi.org/10.1007/s10854-018-0251-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0251-2