Abstract
In the present work, we have investigated structural, dielectric, ac conductivity, magnetic and electromagnetic interference (EMI) shielding effectiveness (SE) performance of Co0.5Ni0.5Fe2O4 nanoparticles and Polyaniline (PANI)/Co0.5Ni0.5Fe2O4 nanocomposites for EMI shielding applications. The Co0.5Ni0.5Fe2O4 nanoparticles were synthesized by solution combustion method. PANI/Co0.5Ni0.5Fe2O4 nanocomposites were prepared by in-situ polymerization method. As-prepared samples were examined by using X-ray diffraction (XRD) and Fourier transform infrared (FT-IR) spectroscopy. The microstructural and composition studies have been performed using Field emission scanning electron microscopy (FE-SEM) and Energy Dispersive X-ray (EDX) analysis. Thermal stability of the composite was analyzed using thermo gravimetric analysis (TGA). Frequency dependence of dielectric and ac conductivity (σac) studies have been undertaken on the Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites in the frequency range 50 Hz–5 MHz. The electrical conduction mechanism in the synthesized samples found to be in accordance with the electron hopping model. Present Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites exhibited hysteretic behavior under the applied magnetic field at room temperature. The maximum values 39.9 and 58.22 dB of SE at 50 Hz were obtained at room temperature for Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites respectively. Present Co0.5Ni0.5Fe2O4 nanoparticles decorated with PANI can be recognized as a promising functional material for the absorbing of electromagnetic (EM) waves due to large amount of dipole polarizations in the polymer backbone and at the interfaces of the Co–Ni ferrite nanoparticles and PANI matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recent advances in technology introduced enormous number of electronic devices into environment. Due to the dense population of electronic systems in the environment, electromagnetic interference (EMI) is the obvious concern and best remedy for this problem is shielding of the electromagnetic radiation. Shielding of electromagnetic field can be done either by reflection or absorption of electromagnetic (EM) waves. The combination of both reflection and absorption for shielding is found to be a better method. Materials with mobile charge carriers (electrons or holes) can effectively shield radiation due to the interaction of mobile charge carriers with electromagnetic fields in the radiation. Materials having higher electrical conductivity are by far the most common materials used for EMI shielding. They function mainly by reflection due to the free electrons in them [1]. Recently, much interest is concentrated on utilizing the flexible polymeric materials and their composites with magnetic materials for EMI shielding applications [2]. Among the flexible polymeric materials, conducting polymers are considered as one of the best material because of its shielding ability due to both from reflection and the absorption [3].
Composites with high electrical conductivity and dielectric constant are most suitable for EMI shielding. Generally, the fillers used for EMI shielding composites are usually divided into two types, namely ferrite particles and metallic particles. As compared to metallic particles, ferrite particles have many important characteristics such as low permittivity, high resonance frequency, high resistivity, low density and good chemical stability [4]. In addition, intrinsically conducting polymers have been studied extensively due to their intriguing electronic and redox properties with numerous potential applications in many fields. Conducting polymers are attractive class of materials similar to metals while retaining flexibility and processability of conventional polymers [5,6,7]. Polyaniline (PANI) is most extensively used conducting polymer due to its appealing properties like stability and comparably ease of processing. In recent years, polymer composites containing magnetic powder have been investigated in numerous research problems for EMI shielding [2, 8, 9].
In the current work, the Co0.5Ni0.5Fe2O4 nanoparticles were prepared by solution combustion method and PANI/Co0.5Ni0.5Fe2O4 nanocomposites were synthesized by in-situ polymerization method. Structural, dielectric, ac conductivity, magnetic and electromagnetic shielding studies have been undertaken on the synthesized samples.
2 Experimental
2.1 Materials
All chemicals and reagents used for synthesis were of analytical grade. Cobalt nitrate, Nickel nitrate, Ferric nitrate, Urea, APS (ammonium per sulfate), HCl and Aniline were used directly without further purification.
2.2 Synthesis of Co0.5Ni0.5Fe2O4 nanoparticles
The Co0.5Ni0.5Fe2O4 nanoparticles have been prepared by solution combustion method. The stoichiometric amounts of Cobalt nitrate, Nickel nitrate and Ferric nitrate as oxidizers and Urea as a fuel were dissolved in distilled water to prepare homogeneous aqueous solution. The above solution containing redox mixture is heated in a muffle furnace maintained at 500 °C till complete combustion. The mixture finally yields porous and voluminous powder containing Co0.5Ni0.5Fe2O4 nanoparticles.
2.3 Synthesis of PANI/Co0.5Ni0.5Fe2O4 nanocomposites
PANI/Co0.5Ni0.5Fe2O4 nanocomposites having ferrite to Aniline monomer ratio of 1:2 were synthesized by in-situ polymerization method [9, 10]. About 1 g of Co0.5Ni0.5Fe2O4 nanoparticles were suspended in a 1 M HCl solution and stirred for half an hour to get well dispersed. Aniline monomer (2 mL) was added to the above suspension and stirred for 30 min. Further, 4.98 g of ammonium per sulfate in 1M HCl solution was then added slowly drop wise to the suspension mixture with a constant stirring. The stirring was continued for 12 h at room temperature. During this period, the color of the mixture was changed to dark green from milky white. The suspension was then filtered and washed with 1M HCl and distilled water. Finally, obtained sample was dried at 60 °C to get PANI/Co0.5Ni0.5Fe2O4 nanocomposites in powder form.
2.4 Characterization
The X-ray diffractograms of the synthesized samples were recorded using Panalytical X-Pert Pro MPD instrument. The samples were scanned in the 2θ range of 10°–70°, with a scanning speed and step size of 5°/min and 0.02°, respectively.
Fourier transform infrared (FTIR) spectra of the samples were recorded in transmission mode using Thermo Nicolet Avatar 370 FTIR spectrophotometer having a resolution 4 cm− 1 in the wave number range 400–4000 cm− 1. Samples were mixed with KBr powder for FTIR measurements. Background correction was made using a blank KBr pellet as a reference.
The morphology of the synthesized samples were analyzed using Field emission scanning electron microscopy (FE-SEM) attached with Energy Dispersive X-ray (EDX) analysis FESEM CARL ZEISS instrument.
Thermogravimetric analysis (TGA) of the sample was carried out by Perkin Elmer Thermal Analysis system with nitrogen as flushing gas. The scanned temperature range was 25–700 °C at a predetermined rate of 20 °C/min.
Dielectric and ac conductivity studies on the prepared samples have been undertaken using impedance analyzer model HIOKI 3532-50 LCR HI TESTER Version 2.3 (frequency range 42 Hz–5 MHz). The measurements were carried out on the samples in the form of compressed pellet at room temperature in the frequency range 50 Hz–5 MHz. Magnetic studies were conducted using vibrating sample magnetometer (Lakeshore VSM 7410).
3 Results and discussion
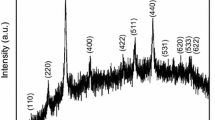
The XRD patterns of Polyaniline, Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites are shown in Fig. 1. XRD pattern of Polyaniline (Fig. 1a) indicates the partially crystalline state with two diffraction peaks centered at around 2θ = 20.7° and 25.1° [11]. Crystallinity of polyaniline is due to the presence of benzenoid and quinoid rings of Polyaniline chain. The major diffraction peaks for Co0.5Ni0.5Fe2O4 nanoparticles (Fig. 1b) are identified at (220), (311), (222), (400), (422), (511), and (440). Among these diffraction peaks, (311) is found to have maximum intensity. The average crystallite size of the samples was estimated by Debye–Scherrer formula \(D=k\lambda /\beta cos\theta\) where, D is the average crystallite size, λ is the wavelength of the X-ray and β is the full width at half maxima (FWHM) of (311) reflection peak. The average crystallite size of the Co0.5Ni0.5Fe2O4 nanoparticles is estimated to be around 47 nm. The presence of combined peaks of both polyaniline and Co0.5Ni0.5Fe2O4 nanoparticles can be clearly observed from Fig. 1c
The FTIR spectra of Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites are shown in Fig. 2. The presence of peaks at 1556 and 1482 cm− 1 (Fig. 2b) confirms the polymerization of aniline [12]. Characteristic peaks of polyaniline are found at 1679, 1556 and 1489 cm− 1. The peaks at 1556 and 1493 cm− 1 corresponds to C=C stretching deformation of quinoid and benzenoid units of PANI, respectively. The peak observed at 1284 cm− 1 attributes C–N stretching of secondary amine in polymer main chain [13]. The bands at 1400–1625 and 1128 cm− 1 of the composites (Fig. 2b) indicates the coupling effect of ferrite and polyaniline [9].
Morphologies of the Polyaniline, Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 composites were recorded using a FESEM and the images of Polyaniline, Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites are shown in Fig. 3. FESEM images clearly indicate that the samples consist of nanosized particles which are agglomerated together.
The elemental composition of PANI/Co0.5Ni0.5Fe2O4 nanocomposites in powder form was determined using EDX analysis. The spectrum of the prepared sample is represented in Fig. 4. EDX measurements on the surface of the sample is used to confirm the components present in PANI/Co0.5Ni0.5Fe2O4 nanocomposites. The peaks of N, O, S, Cl, Fe, Co and Ni elements in EDX spectrum of PANI/Co0.5Ni0.5Fe2O4 composites clearly indicates the presence of Polyaniline and Co0.5Ni0.5Fe2O4 nanoparticles. All the components are distributed homogeneously throughout the tested sample and the average values are given in Table 1.
Figure 5 shows the TGA curve of PANI/Co0.5Ni0.5Fe2O4 nanocomposites. The TGA curve shows 9% weight loss at lower temperature (< 100 °C) due to the vaporization of water in the PANI/Co0.5Ni0.5Fe2O4 nanocomposites. The weight change was not significant and the sample was thermally stable. In the second step there is a weight loss of about 10% in the temperature range 100–400 °C, which is ascribed to the degradation of the polymer chains and PANI has the larger weight loss (about 48%) in the temperature range between 400 and 700 °C. The decomposition temperature of the PANI/Co0.5Ni0.5Fe2O4 nanocomposites was found to depend on the amount of Co0.5Ni0.5Fe2O4 nanoparticles present in the composite [14, 15].
Variation of dielectric constant (ε′) with frequency for Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites at room temperature are shown in Fig. 6. The dielectric constant is a measure of the ability of the material to be polarized. High dielectric constant value in any material is the result of high polarization in that material. The frequency of the applied electrical field induces polarization within the material. Mainly there are four major mechanisms causing polarizations which are electronic, ionic, dipolar and space charge polarization [2, 9]. In lower frequency region, space charge polarization and dipolar polarization plays a major role, where in higher frequency region, electronic and ionic polarizations are prominent [16]. From Fig. 6, it is clear that dielectric constant of Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites decreases with increasing frequency. Present Co0.5Ni0.5Fe2O4 sample was found to exhibit a usual dielectric dispersion behavior. In the lower frequency region, dielectric constant decreases rapidly and the rate of decrease was lower at higher frequency region. Dielectric behavior of this kind can be explained using Maxwell–Wagner model and Koop’s theory [17, 18]. In the lower frequency region, the electrons through hopping between ferrous and ferric ions in the lattice reaches the grain boundaries, accumulation of large number of electrons at grain boundaries produces interfacial polarization in turn leads to high dielectric constant values. As the applied frequency increases, electrons following the applied frequency change their direction, thus the accumulation of electrons at grain boundaries decreases resulting in the low dielectric constant values and also it is due to the fact that the electronic exchange between ferrous and ferric ions not able to follow the applied alternating field at higher frequencies [19].
Frequency dependence of ac conductivity at room temperature for Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites are shown in Fig. 7. The electrical conductivity in ferrites is mainly due to the hopping of electrons between ions of the same element present in more than one valence state and distributed randomly over crystallographic equivalent lattice sites [20]. Due to the activeness of grain boundaries at the lower frequency region there is less hooping of electrons between Fe2+ and Fe3+ ions which leads to the lower ac conductivity. The ac conductivity at lower frequencies is found to be weakly dependent on frequency due to the nonequilibrium occupancy of the trap charges in the PANI matrix [9]. As the frequency increases, conductive grains become more active and cause the increase in hopping of electrons between Fe2+ and Fe3+ ions resulting in an enhancement of ac conductivity. A further amplification of frequency reduces the occupancy of the trap centers by making them available for conduction [14, 21].
M-H curve for Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites at room temperature are shown in Fig. 8. It is clear from the curves that the magnetization in the prepared samples increases as the applied magnetic field increases and attains saturation. The saturation magnetization (Ms) and coercivity (Hc) values of Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites are tabulated in Table 2. The Polyaniline coating causes the decrease in the value of saturation magnetization of Co0.5Ni0.5Fe2O4 nanoparticles from 0.72992 to 0.19874 emu. Decrease in saturation magnetization of Co0.5Ni0.5Fe2O4 nanoparticles by the coating of polyaniline is due to decrease in interaction between the Co0.5Ni0.5Fe2O4 ferrite particles because of increase in particle–particle separation within the PANI matrix [22, 23].
Generally, electromagnetic (EM) shielding by the material is mainly due to three mechanisms: reflection of the wave from the material, absorption of the wave as it passes through the material and multiple reflections of the waves at various interfaces [24]. Multiple reflections can be ignored when the total SE is greater than 10 dB [25], thus total shielding effectiveness (SE) can be obtained by measuring SE due to reflection and absorption losses. The SE of any material is the ratio of incident power to the transmitted power and it is given by,
where, P i and P o are the respective incident and transmitted EM powers. Total shielding effectiveness is given by SE = SE R + SE A . i.e.
and
where, \({\omega _H}=2\pi {f_H}\) is angular frequency, \({\varepsilon _o}\) is the permittivity of free space, \({\mu _r}\) is the relative permeability, t represents sample thickness, \(\delta\) is the skin depth and it is given by \(\delta ={[2/{\omega _H}\mu {\sigma _{ac}}]^{1/2}}\).
Shielding effectiveness values (SE R and SE A ) at room temperature for Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites are calculated using Eqs. (2) and (3). The frequency dependent total SE for the corresponding samples are shown in Fig. 9. The SE values of both Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites are high in lower frequency region and decreases as the frequency increases. The maximum SE values of 39.9 and 58.22 dB were observed for Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites at 50 Hz respectively. The SE value of present PANI/Co0.5Ni0.5Fe2O4 nanocomposites is higher as compared to our previously reported SE value for PANI/ Ni0.5Zn0.5Fe2O4 composites [9]. According to Guan et al. [26] and Li et al. [27], the material can be utilized for electromagnetic shielding purpose in the lower frequency region if the SE is greater than 5 and 15 dB respectively. Present PANI/Co0.5Ni0.5Fe2O4 nanocomposites possess acceptable SE in the studied frequency range. Significant enhancement of SE values for the present PANI/Co0.5Ni0.5Fe2O4 nanocomposites as compared to Co0.5Ni0.5Fe2O4 sample is due to the combined advantage of the presence of Co0.5Ni0.5Fe2O4 ferrite nanoparticles and conducting PANI. The attenuation of EM waves in Co0.5Ni0.5Fe2O4 ferrite nanoparticles is due to the existence of free electrons and material defects. Whereas, in PANI, the EM waves are reduced due to the intrinsic dipole polarization [28].
4 Conclusion
The Co0.5Ni0.5Fe2O4 nanoparticles were synthesized by solution combustion process. Polyaniline (PANI)/Co0.5Ni0.5Fe2O4 nanocomposites were prepared by in-situ polymerization method. Synthesized samples were examined by using XRD, FT-IR, FE-SEM and EDX analysis techniques. Thermal stability of the samples was analyzed using TGA. The electrical conduction mechanism in the Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites is found to be in accordance with the electron hopping model. Both Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites exhibited hysteretic behavior under the applied magnetic field at room temperature. The maximum values 39.9 and 58.22 dB of SE at 50 Hz were obtained at room temperature for Co0.5Ni0.5Fe2O4 nanoparticles and PANI/Co0.5Ni0.5Fe2O4 nanocomposites respectively. The Co0.5Ni0.5Fe2O4 nanoparticles decorated with PANI may be recognized as a promising functional material for the absorbing of electromagnetic (EM) waves due to large amount of dipole polarizations in the polymer backbone and at the interfaces of the Co–Ni ferrite nanoparticles and PANI matrix.
References
D.D.L. Chung, Carbon 50, 3342–3353 (2012)
B.J. Madhu, S.T. Ashwini, B. Shruthi, B.S. Divyashree, A. Manjunath, H.S. Jayanna, Material Science and Engineering B 186, 1–6 (2014)
K. Anil Ohlan, A. Singh, V.N. Chandra, S.K. Singh, Dhawan, J. Appl. Phys. 106, 044305 (2009)
Z.W. Li, Z.H. Yang, L.B. Kong, Procedia Eng. 75, 19–23 (2014)
S.K. Shukla, M. Vamakshi, A. Bharadavaja, A. Shekhar, A. Tiwari, Adv. Mater. Lett. 3, 421–425 (2012)
M.A. Rahman, P. Kumar, D.S. Park, Y.B. Shim, Sensors 8, 118–141 (2008)
K. Gurunathan, A. Vadivel Murugan, R. Marimuthu, U.P. Mulik, D.P. Amalnerkar, Mater. Chem. Phys. 61, 173–191 (1999)
J. Wu, D.D. Chung, J. Electron. Mater. 37, 1088–1094 (2008)
B.J. Madhu, M. Gurusiddesh, T. Kiran, B. Shruthi, H.S. Jayanna, J. Mater. Sci: Mater Electron 27, 7760–7766 (2016)
Y. Yan Wang, Q. Huang, Q. Wang, L. He, Chen, Appl. Surf. Sci. 259, 486–493 (2012)
M. Khairy, M.E. Gouda, J. Adv. Res. 6, 555–562 (2015)
M. Khairy, Synth. Met. 189, 34–41 (2014)
J. Jiang, L. Li, F. Xu, Mater. Sci. Eng. A 456, 300–304 (2007)
C.L. Yuan, Y.S. Hong, C.H. Lin, J. Magn. Magn. Mater. 323, 1851–1854 (2011)
G.D. Prasanna, R.L. Ashok, V.B. Prasad, H.S. Jayanna, J. Compos. Mater. 49, 2649–2657 (2015)
J. Chand, M. Singh, J. Alloy. Compd. 486, 376–379 (2009)
K.W. Wagner, J. Amer. Phys. 40, 317 (1973)
C.G. Koops, Phys. Rev. 83, 121–124 (1951)
S. Joshi, M. Kumar, J. Supercond. Nov. Magn. 29, 1561–1572 (2016)
K.M. Batoo, M.S. Ansari, Nanoscale Res. Lett. 7, 112 (2012)
M. Younas, M. Nadeem, M. Atif, R. Grossinger, J. Appl. Phys. 109, 093704 (2011)
J. Jiang, L. Li, F. Xu, J. Phys. Chem. Solids 68, 1656–1662 (2007)
X. Batlle, A. Labarta, J. Phys. D 35, R15-R42 (2002)
R. Kumar, S. Kumari, S.R. Dhakate, Appl. Nanosci. 5, 553–561 (2015)
N.C. Das, D. Khastgir, T.K. Chaki, A. Chakraborty, Compos. A 31, 1069–1081 (2000)
H. Guan, S. Liu, Y. Duan, J. Cheng, Cement Concr. Compos. 28, 468–474 (2006)
N. Li, Y. Huang, F. Du, X. He, X. Lin, H. Gao, Y. Ma, F. Li, Y. Chen, P.C. Eklund. Nano Lett. 6, 1141–1145 (2006)
W. Wang, S.P. Gumfekar, Q. Jiao, B. Zhao, J. Mater. Chem. C 1, 2851–2859 (2013)
Acknowledgements
Authors wish to acknowledge Central college, Bangalore for XRD analysis and SAIF-IITM for VSM facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gurusiddesh, M., Madhu, B.J. & Shankaramurthy, G.J. Structural, dielectric, magnetic and electromagnetic interference shielding investigations of polyaniline decorated Co0.5Ni0.5Fe2O4 nanoferrites. J Mater Sci: Mater Electron 29, 3502–3509 (2018). https://doi.org/10.1007/s10854-017-8285-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-8285-4