Abstract
A modified spray pyrolysis technique was employed for the deposition of Cu2CoSnS4 (CCTS) thin films. X-ray diffraction study showed that the CCTS thin films exhibit stannite structure with preferred orientation along (112) plane. The phase purity of sprayed CCTS films was confirmed from Raman analysis, as the most intense peak is observed at 320 cm−1 corresponding to A1 mode. With increase in substrate temperature the grains over the surface of the CCTS films become more distinct. The EDAX spectrum revealed the presence of different constituent elements such as, Cu, Co, Sn and S for the films deposited at 325 °C. The optical absorption study showed that the energy band gap values of the CCTS films are decreasing from 1.70 to 1.46 eV with respect to increase in substrate temperature from 275 to 325 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In order to fulfill the demand for green energy through photovoltaics much emphasis is needed in the development of new materials which can harvest solar energy for electricity generation [1].The development of cost effective thin film solar cells (TFSC) based on nontoxic, and earth abundant materials, requires the exploration of new photoactive materials. Cu2CoSnS4 (CCTS) is a compound semiconductor, with optimal band gap value in the range 1.46–1.61 eV, which suggest its high potential application in the photovoltaic field for the development of efficient TFSC. In addition, it offers direct band gap that reduces the material need in comparison to other indirect band gap materials like crystalline silicon [2]. CCTS has stannite structure which crystallizes in the tetragonal space group \({\text{I}}\bar {4}2\) m. In the tetragonal unit cell all three metal atoms are tetrahedrally surrounded by sulfur atom, and each sulfur atom is surrounded by one Co, one Sn, and two Cu atoms [3]. In stannite structure, Co and Sn atoms are in the same layer alter their positions every other layer. A layer of Cu alternates with a layer of Co and Sn [4]. The CCTS semiconductor has been synthesised by different techniques such as, hydrothermal [5], solvothermal [6,7,8], sol–gel [9], hot injection [10], solid state reaction [11] electrospinning [12,13,14], high temperature route [15, 16], melt and anneal process [17, 18]. The most of the research in case of CCTS has been devoted in the synthesis of nanocrystals. With an intention of practical application in photovoltaic devices, the CCTS thin films need to be thoroughly studied. Among the diverse methods available for synthesis of thin films the spray pyrolysis technique offers certain advantages such as easy handling, non vacuum and large area deposition [19].
This paper represents effect of temperature on structural, morphological and optical properties of spray deposited CCTS thin films. The CCTS thin films are deposited with PID logic using Arduino platform. The working temperature range is maintained with precision of (± 2) °C related to operating temperature set by user. The Arduino platform is also utilized to drive DC motor which provides mechanized movement of the spray nozzle to facilitate the regular deposition of thin films.
2 Experimental details
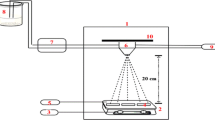
Figure 1 shows the schematic of the spray pyrolysis unit used for deposition of CCTS thin films. The deposition of CCTS thin films was carried out using precursors, 0.05 M CuCl2·2H2O (Cupric Chloride) for copper, 0.025 M CoCl2·6H2O (Cobalt Chloride hexahydrate) for cobalt, 0.025 M SnCl4·5H2O (Stannic Chloride) for tin, and thiourea (CH4N2S) was used in excess than stoichiometry as a source of sulfur. Methanol was used as solvent to prepare precursor solution. The 0.05 M CuCl2·2H2O solution was prepared by dissolving 0.17 g of CuCl2·2H2O powder in 20 mL of methanol. The 0.025 M CoCl2·6H2O solution was prepared by dissolving 0.12 g of CoCl2·6H2O powder in 20 mL of methanol. The 0.025 M SnCl4·5H2O solution was prepared by dissolving 0.17 g of SnCl4·5H2O powder in 20 mL of methanol. The appropriate amount of thiourea powder was dissolved in 20 mL of methanol, to achieve the ratio of cations to anions as 1:5 in the precursor solution. The molarity of thiourea was chosen in excess to avoid the loss of sulfur at elevated temperature [20]. The mixing of all the cationic solutions together in proper order resulted into brown colored solution with pH ≈ 1. In this cationic solution, 20 mL of thiourea was added drop wise under vigorous stirring for 30 min. After addition of complete 20 mL thiourea to cationic solution, the final color of the precursor solution was observed to be dark blue with pH ≈ 3. This solution was used for the deposition of CCTS thin films. To facilitate the regular deposition of thin films mechanized movement has been employed to spray nozzle at a frequency of 0.16 Hz. The atomization of precursor solution into fine spray droplets was carried out by compressed filtered air with pressure kept at ≈1 bar. The 80 mL of precursor solution with a flow rate of 5 mL/min was sprayed onto soda lime glass (SLG) substrates which were preheated using temperature control arrangement. The working temperature range was chosen to be (±2) °C of threshold set point entered by user with the help of PID based logic in Arduino platform. The deposition temperature was chosen in the range 275–375 °C with an interval of 50 °C.
Surface profiler (Ambios, XP-I stylus profiler, USA) was used to carry out the thickness of measurement of the films. The structural properties of CCTS films were studied by using X-ray diffractometer (XRD) operated at 40 kV, 30 mA with Cu Kα radiation (λ = 1.5406 Å). The Raman analysis was carried out with the help of Renishaw inVia micro-Raman spectrometer at excitation wavelength of 633 nm of He–Ne laser as excitation source. The surface morphology of the films was studied by scanning electron microscopy (SEM) (JSM-6360, JEOL, Japan). The energy dispersive X-ray analysis (EDAX) of the film was recorded using EDX detector (Oxford Instruments, UK). UV–Visible spectrophotometer (UV 1800, Shimadzu, Japan) was used to record UV–visible absorbance spectra of the films.
3 Results and discussion
3.1 CCTS formation mechanism
The precursor solution for the formation of CCTS consists of dissolved metal chlorides and thiourea in methanol. The metal ions in the precursor solution attach to thiourea via sulfur atom to form metal–thiourea complex \(~{[{\text{CuCoSn(CS(N}}{{\text{H}}_2}{)_2}{)_{\text{n}}}]^{{\text{m+}}}}\). During spray pyrolysis heat treatment breaks the C–S bond present in the Cu–Co–Sn–thiourea complex, and metal sulfide i.e. Cu2CoSnS4 is released [21,22,23,24,25]. The dissolutions of metal chlorides and thiourea in solvent will result into following reactions
The formation of metal–thiourea complex is represented by
The overall chemical reaction for CCTS formation on SLG substrates is given by
Thiourea used in the precursor solution not only acts as a source of sulfur but also it functions as reducing agent of divalent Cu ion to monovalent Cu ion [26].
3.2 Thickness measurement
The thickness of the CCTS films deposited at different substrate temperatures such as 275, 325 and 375 °C is found to be 527, 338 and 283 nm, respectively. The relatively larger value of thickness obtained at deposition temperature 275 °C is ascribed to incomplete thermal decomposition of spray droplets for the set temperature.
3.3 Structural analysis
Figure 2 shows XRD patterns of CCTS thin films deposited at different substrate temperatures. For the CCTS film deposited at substrate temperature of 275 °C, the intensity of (112) plane is weak as compared to film deposited at 325 °C. This may be due to insufficient thermal energy needed for decomposition of precursor solution. For the film deposited at substrate temperature of 325 °C, intensity of (112) plane is strong as well as the weak reflections at 2θ values of 47.50° and 56.40° are observed corresponding to (204) and (312) planes, respectively. This is due to the sufficient amount of thermal energy needed for thermal decomposition of precursor solution onto the glass substrates. Therefore, this substrate temperature is suitable for optimum growth condition for film formation [27]. For the film deposited at substrate temperature of 375 °C, the intensity of (112) plane decreases and the reflections corresponding to (204) and (312) planes disappears. This is due to the complete decomposition of precursor solution in air before reaching towards the glass substrate, which results into the formation of less adherent powdery film onto the glass substrate. Also, at such a higher substrate temperature, less mass transport of precursor species onto glass substrate result into the decrease in film thickness [28].
Comparing the XRD spectra of CCTS thin films to standard JCPDS Card No: 00-026-0513, it is confirmed that the spray deposited CCTS thin films exhibit stannite structure which has tetragonal unit cell. The average crystallite size (D) is calculated by Scherrer’s Eq. [29].
where k is the shape factor, usually taken as 0.9 for spherical crystallites, λ is the wavelength of X-ray used, β is FWHM of the peak under consideration and θ is the Bragg’s angle. Table 1 shows the values of diffraction angle, interplanar spacing dhkl, FWHM and crystallite size for (112) plane for the films deposited at different substrate temperatures. It is observed that with increase in substrate temperature from 275 to 375 °C, the full-width at half-maximum (FWHM) of (112) plane becomes narrower which represents the better crystallites formation in the CCTS films.
3.4 Raman analysis
XRD patterns of secondary phase like Cu2SnS3 (CTS) and CCTS show close resemblance which limits the discrimination of CTS and CCTS structures by X-ray diffraction alone. Therefore, Raman analysis is carried out to confirm the phase purity of spray deposited CCTS thin films. Figure 3 shows a typical Raman spectrum for CCTS thin films deposited at 325 °C in the range of 200–450 cm−1. The most intense peak is observed at 320 cm−1, this can be allotted to the symmetry mode A1. It is mainly due to vibrations of S anions. The absence of peak at 350 cm−1 denies the presence of metal polysulfide impurities of the types SnS2 and CuCoS2 in CCTS thin films [30].
3.5 Morphology of the films
Figure 4 put on view of SEM micrographs of CCTS thin films deposited at different substrate temperatures. At deposition temperature 275 °C morphology of the film is found to be nonuniform that represents the lack of distinct grains along with few voids over the surface. The grains in the films deposited at higher temperatures such as 325 and 375 °C, are appeared relatively larger than the grains seen in film at substrate temperatures 275 °C. For the films deposited at 325 °C the surface of the film seems to be flat with the evidence of voids over the film surface. As agglomeration is post nucleation process [31], the availability of nucleation centres resulted into large cauliflower like clusters at some region over the film surface. More compact morphology is observed for the films deposited at substrate temperatures 375 °C. The surface of the film is observed to be nonuniform which consists of some voids.
3.6 Compositional analysis
EDAX spectrum of the CCTS films deposited at temperature 325 °C is shown in Fig. 5. The Peaks corresponding to different constituent elements like Cu, Co, Sn, and S are clearly seen in the EDAX spectrum of the film. The composition ratio Cu:Co:Sn:S is found to be 2.13:1:1.91:5.39. EDAX spectrum reveals that the as deposited CCTS films are slightly Sn and S rich. The deviation from stoichiometry of the sulfur can be attributed to higher concentration of thiourea in the precursor solution.
3.7 Optical properties
The relation between absorption coefficient α and incident photon energy hν is given by Eq. [32].
where A is proportionality constant, Eg is optical band gap energy, n is an index representing the optical absorption process and it takes values equal to 1/2 or 2 for the direct and indirect allowed transitions respectively. As CCTS is direct band gap material, for direct allowed transitions n takes value equal to 1/2. The optical band gap of CCTS thin films can be obtained by extrapolating the linear portion of the plots \(~{(\alpha{\text{h}}\nu)^2}\) against \({\text{h}\nu}\) at \(\alpha=0\) as shown in Fig. 6. The energy band gap values of as deposited CCTS films are found to be decrease with increase in substrate temperature. The decrease in band gap values is ascribed to increase in crystallite size of the CCTS films deposited at different substrate temperatures [33, 34]. The energy band gap values of the CCTS films deposited at different substrate temperatures such as 275, 325 and 375 °C was found to be 1.70, 1.53 and 1.46 eV, respectively. The obtained band gap values for CCTS films are quite close to the materials used for solar cell application [35].
4 Conclusions
Thin films of CCTS have been deposited by spray pyrolysis technique. The XRD studies revealed that the spray deposited thin films exhibit stannite structure with preferred orientation along (112) plane. The Raman analysis confirms the phase purity of spray deposited CCTS films, as the most intense peak is observed at 320 cm−1 corresponding to A1 mode. The surface morphology study shows that the grains over the surface of the CCTS films become more distinct with increase in substrate temperature. The EDAX spectrum revealed the presence of elements Cu, Co, Sn and S. From the EDAX spectrum, it is observed that the elemental composition of CCTS films deposited at 325 °C is found to be Sn and S rich. The optical absorption study shows that the energy band gap values of as deposited films decrease with increase in substrate temperature. The spray deposited films at 325 °C have band gap close to optimal band gap of 1.5 eV having compact and densely packed morphology, seems to be potential candidates for absorber layer in cost effective thin film solar cells.
Change history
07 October 2017
The original version of this article unfortunately contained an error in Sect. 3.3 Structural analysis Equation (7). The corrected version is published with this erratum (Eq. 7).
References
F. López-Vergara, A. Galdámez, V. Manríquez, G. González, Solid State Sci. 49, 54–60 (2015)
Y. Xie, C. Zhang, G. Yang, J. Yang, X. Zhou, J. Ma, J. Alloys Compd. 696, 938–946 (2017)
W. Schfifer, R. Nitsche, 9, 645–654 (1974)
A. Khare, B. Himmetoglu, M. Johnson, D.J. Norris, M. Cococcioni, E.S. Aydil, J. Appl. Phys. 111, 083707 (2012)
C. An, K. Tang, G. Shen, C. Wang, L. Huang, Y. Qian, Mater. Res. Bull. 38, 823–830 (2003)
Y. Cui, R. Deng, G. Wang, D. Pan, J. Mater. Chem. 22, 23136–23140 (2012)
K. Mokurala, S. Mallick, P. Bhargava, J. Power Sources 305, 134–143 (2016)
J.Y. Chane-Ching, a Gillorin, O. Zaberca, A. Balocchi, X. Marie, Chem. Commun 47, 5229–5231 (2011)
B. Murali, M. Madhuri, S.B. Krupanidhi, Cryst. Growth Des 14, 3685–3691 (2014)
A. Gupta, K. Mokurala, A. Kamble, S. Shankar, S. Mallick, P. Bhargava, AIP Conf. Proc. 1665, 140022 (2015)
F. López-Vergara, A. Galdámez, V. Manríquez, P. Barahona, O. Peña, J. Solid State Chem. 198, 386–391 (2013)
F. Ozel, J. Alloys Compd. 657, 157–162 (2016)
F. Ozel, A. Yar, E. Aslan, E. Arkan, A. Aljabour, M. Can, I.H. Patir, M. Kus, M. Ersoz, ChemNanoMat 1 477–481 (2015)
M.K. Gonce, E. Aslan, F. Ozel, I.H. Patir, ChemSusChem 9, 600–605 (2016)
M. Benchikri, O. Zaberca, R. El Ouatib, B. Durand, F. Oftinger, A. Balocchi, J.Y. Chane-Ching, Mater. Lett. 68, 340–343 (2012)
O. Zaberca, A. Gillorin, B. Durand, J.Y. Chane-Ching, J. Mater. Chem. 21, 6483 (2011)
L.D. Gulay, O.P. Nazarchuk, I.D. Olekseyuk, J. Alloys Compd. 377, 306–311 (2004)
M. Quintero, E. Moreno, S. Alvarez, J. Marquina, C. Rincón, E. Quintero, P. Grima, J. Heano, M.A. Macías, Rev. Lat. Met. Mater. 4, 28–38 (2014)
S.P. Desai, M.P. Suryawanshi, S.M. Bhosale, J.H. Kim, A.V. Moholkar, Ceram. Int. 41, 4867–4873 (2015)
G. Ghosh, A. Biswas, A. Thangavel, R., and Udayabhanu, RSC Adv 6, 96025–96034 (2016)
R.D. Angelo, C.L. Azanza Ricardo, A. Mittiga, P. Scardi, M. Leoni, J. Sol–Gel Sci. Technol 72, 490–495 (2014)
T.K. Chaudhuri, D. Tiwari, Sol. Energy Mater. Sol. Cells 101, 46–50 (2012)
G. Rajesh, N. Muthukumarasamy, E.P. Subramaniam, S. Agilan, D. Velauthapillai, J. Sol–Gel Sci. Technol. 66, 288–292 (2013)
S. Dias, B. Murali, S.B. Krupanidhi, Mater. Chem. Phys. 167, 309–314 (2015)
Z. Seboui, A. Gassoumi, N. Kamoun-turki, Mater. Sci. Semicond. Process. 26, 360–366 (2014)
S. Ikeda, M. Nonogaki, W. Septina, G. Gunawan, T. Harada, M. Matsumura, Catal. Sci. Technol. 3, 1849–1854 (2013)
A. Kumar, P. Rajaram, Mater. Sci. Eng. B. 172, 37–42 (2010)
A.A. Mane, M.P. Suryawanshi, J.H. Kim, A.V. Moholkar, J. Colloid Interface Sci. 483, 220–231 (2016)
L. Shi, Y. Li, H. Zhu, Q. Li, Chempluschem 79, 1638–1642 (2014)
F. López-Vergara, J. Chil. Chem. Soc. 4, 2131–2135 (2013)
G. Nichols, S. Byard, M.J. Bloxham, J. Botterill, N.J. Dawson, A. Dennis, V. Diart, N.C. North, J.D. Sherwood, J. Pharm. Sci. 91, 2103–2109 (2002)
B. Murali, S.B. Krupanidhi, J. Appl. Phys. 114, 144312 (2013)
P.E. Agbo, Adv. Appl. Sci. Res. 3, 599–604 (2012)
H.B. Lee, Y.M. Yoo, Y.H. Han, Scr. Mater. 55, 1127–1129 (2006)
A. Ghosh, D.K. Chaudhary, A. Biswas, T. Rajalingam, G. Udayabhanu, RSC Adv. 6 115204–115212 (2016)
Acknowledgements
Authors wish to acknowledge “CENTRAL SURFACE ANALYTICAL FACILITY of IIT Bombay” for providing the SEM Images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maldar, P.S., Mane, A.A., Nikam, S.S. et al. Temperature dependent properties of spray deposited Cu2CoSnS4 (CCTS) thin films. J Mater Sci: Mater Electron 28, 18891–18896 (2017). https://doi.org/10.1007/s10854-017-7842-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7842-1