Abstract
Solid polymer electrolytes (SPEs) based on polyvinyl alcohol (PVA) and sodium triflate (NaTf) have been prepared by solution cast technique. X-ray diffraction was performed for structural analysis. The decrease of intensity of crystalline peaks of PVA upon addition of NaTf salt reveals the increase of amorphous domain in SPEs. Impedance plots (Zi vs. Zr) shows that the electrolyte samples have a smaller bulk resistance. The highest achieved room temperature DC conductivity is 7.39 × 10−5 S/cm for the sample incorporated with 30 wt% of NaTf. The influence of lattice energy of sodium salts on DC conductivity is discussed. It was appeared that the lattice energy of salts significantly affects the conductivity behavior of polymer electrolytes. High DC conductivity can be achieved for polymer electrolytes incorporated with low lattice energy salts. The pattern of DC ionic conductivity against 1000/T is follows Arrhenius equation. The highest conducting composition has the lowest activation energy (0.109 eV). The DC conductivities calculated from the complex impedance plots are close to those obtained from the plateau region of AC conductivity spectra. Transport parameters such as density of mobile ions (Na+), mobility, and diffusion coefficient were determined for the samples using the Rice and Roth models. The dielectric analysis suggests non-Debye type of relaxation of ions in the present work.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ion conducting polymer electrolytes (ICPEs) are attractive due to their wide applications in electrochemical devices such as high energy solid sate batteries, dye-sensitized solar cells, fuel cells, and supercapacitors [1]. The advantages of (ICPEs) are long lifetime, safety, light weight, mechanical flexibility, high corrosion resistance, and easy processing [2, 3]. The main obstacle in front of (ICPEs) is their low ionic conductivity at ambient temperature [4]. Many efforts have been devoted to alter the ionic conductivity of polymer electrolytes. Different salts have been complexed with various host polar polymers [5, 6]. Polyvinyl alcohol (PVA) is a potential polar polymer which is used in the preparation of solid polymer electrolytes (SPEs). The potential use of PVA in SPEs is related to its good dielectric strength, high transparency, low cost, and excellent dopant-dependent electrical properties [5, 6]. PVA has several hydroxyl (OH) groups on its backbone which assists the formation of polymer electrolytes with inorganic salts through hydrogen bonding [7]. The intensive and extensive survey of literature reveals that few attempts were made to develop solid polymer electrolytes based on PVA complexed with sodium salts [8,9,10]. Study of conduction mechanism in polymer based electrolytes has been aimed to understand the nature of ion transport in these materials [11]. Attempts to achieve a higher DC conductivity at room temperature between different salts and different polymers are still in progress. Extensive research has been carried out on Li salts complexed polymer electrolytes. However, only a few attempts have been made on polymer electrolytes containing sodium salts. Sodium is available in abundance at a cheaper cost than lithium. Furthermore, the softness of these materials makes it easier to achieve and maintain contact with other components in the battery [12]. To our knowledge, SPEs based on PVA incorporated with sodium triflate (NaTf) has not been reported. In this work films of SPE based on PVA:NaTf were examined by X-ray diffraction and impedance spectroscopy for structural and electrical characterization. Furthermore transport parameters have been calculated to understand the ionic conduction mechanism in the present polymer electrolyte films.
1.1 Experimental details
1.2 Raw materials
The chemical used in this study were polyvinyl alcohol (PVA) (98–99% hydrolyzed, high molecular weight) supplied by Alfa Aesar, and sodium triflate (NaCF3SO3) with a molecular weight 172.06 g/mol provided by Sigma-Aldrich. Double-distilled water was used as a solvent. These materials used without further treatment or purification.
1.3 Sample preparation
In this work, solid polymer electrolytes based on PVA were prepared by the conventional solution cast technique. One gram of PVA was dissolved in 50 mL of distilled water at 90 °C. The solution was stirred continuously with a magnetic stirrer for several hours until the PVA powder has completely dissolved, to obtain clear viscose solutions. After that, the PVA solution was left to cool down to room temperature. To these sets of solution 10–30 wt% of NaTf was added separately with continues stirring until homogeneous solutions were obtained. The solutions were then cast into different plastic Petri dish and left to dry at ambient temperature (303 K) to allow complete evaporation of the solvent. The films were peeled and kept in desiccators with silica gel desiccant for further drying process. Table 1 shows the concentration of the prepared solid polymer electrolytes based on PVA.
1.4 Characterization
X-Ray Diffraction XRD patterns were recorded using a Siemens D5000 X-ray diffractometer with operating current and voltage of 40 mA and 40 kV, respectively. The samples were scanned with a beam of monochromatic CuKα X-radiation of wavelength (λ = 1.5406 Ǻ), and the glancing angles X-ray diffraction was in the range of 5° ≤ 2θ ≤ 80° with a step size of 0.1°.
The impedance of the samples was measured using the HIOKI 3531-Z Hi-tester in the frequency range 50 Hz to 1 MHz and in the temperature range 303–363 K. The films were mounted on the conductivity holder with blocking stainless steel electrodes of diameter 2 cm.
2 Results and discussion
2.1 XRD analysis
In this study X-ray diffraction (XRD) was performed to reveal the effect of NaTf salt on the crystalline structure of PVA. Figure 1 represent the XRD pattern of pure NaTf salt; it is clear that the crystalline diffraction peaks of pure NaTf salt can be detected at 2θ = 8.50°, 9.95°, 16.80°, 22.15°, 26.25°, 32.90°, 35.60° and 40.90° [13]. Figure 2 illustrates the XRD pattern of pure PVA film. It can be observed that pure PVA film exhibits two characteristic peaks at 19.5° and 39.5°. These peaks can be ascribed to semi-crystalline nature of PVA membrane [14, 15]. The intramolecular and intermolecular hydrogen bonds are responsible for the semi-crystalline structure of PVA. These bonding can be formed between the molecules in individual monomer unit, as well as between different monomer units, respectively [16].
Figure 3 depicts the XRD pattern of SPEs based on PVA:NaTf. It is obvious that the intensity of the crystalline peaks of pure PVA (Fig. 2) decreased with increasing salt concentration. No peaks corresponding to NaTf salt were observed in the XRD pattern of solid polymer electrolyte samples, indicating a complete dissociation of NaTf salt in the polymer matrix [17]. The insets of Fig. 3 show that the position of the main crystalline peak of PVA is shifted. The crystalline peak at 2θ = 39.5° as observed for pure PVA in Fig. 2 almost disappeared in the doped samples (see Fig. 3). The broadening, shifting and lowering the relative intensity of PVA’s diffraction peaks on the incorporation of the NaTf salt can be ascribed to the disruption of hydrogen bonding between amino groups and hydroxyl groups in PVA polymer [14]. Thus the occurrence of complexation between the dissociated salt and the polymer matrices causes the amorphous enhancement in the SPEs samples [18]. These results reveal that NaTf salt has a significant effect on the crystalline phases of PVA solid polymer electrolytes.
2.2 Impedance analysis
Impedance spectroscopy is a well-known method for the characterization of ion dynamics in polymer electrolyte systems. The impedance spectroscopy measurements were utilized in the present solid polymer electrolyte to depict the electrolyte conductivity and its frequency dependent behavior. Figure 4 shows the Cole–Cole plots (Nyquist plots) for pure PVA and PVA:NaTf based polymer electrolytes at room temperature. The complex impedance plots show a high-frequency semi-circle and a low-frequency spike for low salt concentration. The incomplete semi-circle observed at high frequency can be ascribed to the parallel combination of bulk resistance (due to migration of ions) and bulk capacitance (due to immobile polymer chains), whereas the low-frequency spike is due to the effect of electrode–electrolyte interface (i.e. space charge polarization effect) and can be represented by constant phase element [19]. Thus in terms of equivalent circuits the high frequency semicircle can be represented as a parallel combination of a resistor due to ion migration and a capacitor due to the polarization of polymer chains. The centers of the semi-circles are found to be below the Zr-axis. This suggests that the relaxation of ions in the present samples is non-Debye type. It is obvious that as the NaTf concentration increases; the diameter of the high frequency semi-circle decreases. The disappearance of high frequency semi-circle for 30 wt% of NaTf, indicates that the resistive component of the polymer is prevailed and thus the total conductivity is mainly due to the mobile ions inside the polymer matrix [20, 21]. The inset of the impedance plots shows that how the bulk resistance (R b ) can be calculated at low and high salt concentrations. It is interesting to note that R b of pure PVA decreases continuously with increasing NaTf concentration.
2.3 Ion transport study
The DC ionic conductivity (σ dc ) values of SPEs samples are calculated from the following Eq. [22]:
where L and A are the thickness and area of the SPE film, respectively. The significant decrease of R b upon increasing the NaTf salt concentration may be due to the increase in the number of mobile charge carriers [23]. The room temperature conductivity of pure PVA is found to be 1.64 × 10−8 S/cm, which is comparable with the value reported by Bhargav et al. [8], for pure PVA. It can be seen from Table 2 that the DC conductivity of solid polymer electrolyte samples increases with increasing NaTf concentration. This can be attributed to the increase of mobile ions. An increase in charge carrier concentration would lead to an increase in DC conductivity which can be more understood through the following general expression for the DC conductivity at ambient temperature [24,25,26],
where n i is the charge carrier density, q is 1.69 × 10−19 C, and µ i is the mobility of the ions. Different factors could manipulate the ionic DC conductivity such as ionic conducting charge species concentration, cationic- or anionic-type charge carriers, the mobility of charge carriers, and the temperature [27]. Thus, the rapid increase in DC conductivity at 30 wt% of NaTf can be explained by an increase in charge carrier’s concentration, because more salt is added at room temperature [25, 26]. At the same time the XRD results informed us that amorphous domain increased. This may increase the ionic mobility. Thus the mobility of the samples must be calculated. The highest conductivity at ambient temperature is found to be 7.39 × 10−5 S/cm for 30 wt% of NaTf added salt. The DC conductivity reported in literature for PVA incorporated with different sodium salts are 1.02 × 10−5, 1.12 × 10−6 and 2.012 × 10−8 S/cm for 30 wt% of sodium iodide (NaI), sodium bromide (NaBr), and sodium fluoride (NaF) respectively [8,9,10]. Thus the DC conductivity achieved in the present work is higher than those reported in literature. It was established that the dissociation of inorganic salts in macromolecular solids depends on lattice energy of salts and the dielectric constant of the host polymer [28]. Thus the DC conductivity can be discussed on the bases of lattice energy of salts. The lattice energy of NaTf is 650 kJ/mol and is smaller than the lattice energies of NaF, NaBr and NaI which are 923, 747 and 704 kJ/mol, respectively [28]. From the above discussion it is understood that in addition to dielectric constant of host polymers the lattice energy of salts significantly affects the conductivity behavior of polymer electrolytes.
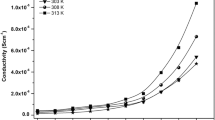
In order to understand the ionic conduction mechanism in PVA/NaTf polymer electrolyte films, the temperature-dependent conductivity measurements were performed. Figure 5 shows the logarithmic plot of dc conductivity log (σ) versus 1000/T for all samples. The linear behavior of DC conductivity (Fig. 5) suggests that ionic conduction mechanism is thermally activated process and follows Arrhenius model [29]. According to Arrhenius the conductivity can be expressed as:
where σ o is a pre-exponential factor, E a is the activation energy, K B Boltzmann constant, and T is absolute temperature in Kelvin. In Eq. 2, the activation energy E a has been assigned as the energy acquired by an ion to free itself from the localized state [30]. The contribution of polymeric chain movements in ionic conduction process cannot be ignored in Arrhenius model [29]. The increase of vibrational motion of the polymer backbone and side chains, at high temperatures brings the coordination sites closer to one another enabling the ions to hop from one site to another site with lesser energy required [26]. Furthermore the increase of amplitude of vibration of the polymer chains at high temperatures increases the fraction of free volume and this resulted in the enhancement of ionic mobility. This is related to the fact that polymer segmental motion provides a pathway for ions to move easily [31, 32].
The values of activation energy (E a ) for all the samples are listed in Table 2. The value of E a achieved in the present work for pure PVA is in good agreement with that reported by other researchers [33]. It is clear from Table 2 that activation energy decreases with increasing NaTf salt concentration. This indicates a decrease in energy barrier for ion migration process. The dominance of amorphous fraction at high NaTf concentration (see Fig. 2) facilitates the ionic motion in the polymer network [4], and thus a lower activation energy. Also from Table 2 it was shown that the highest conductivity polymer electrolyte has the lowest activation energy.
It is well reported that DC conductivity is related to the presence of free charges in polymer electrolyte systems. Whereas, the AC conductivity belongs to the trapped charges in the defect levels which can be activated at high frequency region [34]. Thus the plateau regions in AC conductivity spectra which do not show any dispersion with frequency can be use to estimate the DC conductivity. The typical plot of AC conductivity for all samples is shown in Fig. 6. The spectra consist of two well-defined regions at low salt concentration; the plateau region corresponds to dc conductivity, and one dispersive region. Appearance of spike or tail at low frequency for SPE 20 and SPE 30 samples are attributable to electrode polarization effects [3, 21]. The disappearance of dispersion region in the AC spectra of SPE30 sample is related to the accumulation of free ions at the electrode–electrolyte interface which decreases the number of mobile ions. At higher frequencies, the phase change of AC electric field is too short for the charging to occur, and thus the ac conductivity is appeared as frequency-independent value, which is equal to the DC conduction [35]. From the comparison of impedance plots and AC conductivity spectra it is clear that dispersion regions of AC spectra are corellated with the high frequency semicircle of impedance plots. In our previous [36, 37], works this correlation is well established. The DC conductivity of the pure and doped PVA polymer electrolyte films has been estimated from the intersection of extrapolated plateau regions on AC conductivity axis (see the inset of Fig. 6). The obtained values of DC conductivity were listed in Table 2. The DC conductivity values estimated from AC conductivity spectra coincides well with those calculated from the bulk resistance (R b ).
The transport parameters for PVA:NaTf solid polymer electrolytes were calculated using Rice and Roth model [38]. This model hypothesized that if an ionic carrier of mass m, received an energy E a , it will excited from the localized ionic states to another state with a velocity, v [39]. The velocity of mobile ions can be determined from the following equations,
where l is mean free path between two complexation sites, and τ is the time taken by ions to travel the mean free path. In this study, the short spacing distance (mean free path) between OH groups of PVA is taken to be 2.15 °A [40]. From the Rice and Roth model, the number of mobile ions was calculated usinf the following equation,
Here, Z is valency of ion, and e is electron charge. Once the ionic carrier density achieved, the ionic mobility μ, and diffusion coefficient D, of the samples can also be calculated using the following expressions [41]:
and
All the calculated transport parameters of PVA:NaTf samples at ambient temperature are presented in Table 3. It is obvious that the carrier density increases with increasing NaTf concentration. As well as, the increase of mobility can be observed with increasing salt concentration. This can be related to the increase of amorphous fraction at high salt concentration. It was confirmed that crystalline regions in SPEs impede ion movement by blocking the paths to ions but the increase of amorphous region means an increase in the free volume. The increase in free volume would facilitate the motion of ionic carriers [42]. The XRD results strongly support this interpretation. These results confirm that the conductivity is controlled by both of carrier density and mobility. On the other hand, the increase in diffusion of ions in the polymer matrix will also help to increase the conductivity value. Thus, the highest conductivity sample (SPE 30) possesses the highest diffusion coefficient value of 3.29 × 10−10 cm2s−1 at ambient temperature.
2.4 Dielectric spectra analysis
The dielectric spectroscopy investigation has been proven to be a powerful technique to understand the ion transport mechanisms. The dielectric data were analyzed using complex dielectric constant (ɛ *) and complex electric modulus (M *). Figure 7 represent the dielectric constant (ɛ’) against frequency for pure and doped PVA film, at room temperature. The values of ɛ’ were calculated using the following Eq. [24, 43],
where ω is the angular frequency, and C o is vacuum capacitance (C o = ɛ o A/d, where ɛ o is a free space permittivity). The value of ɛ’ is large at low frequencies, while almost plateau at higher frequencies. At low frequencies, electrode polarization occurs due to the accumulation of free mobile ions at the electrode–electrolyte interface, and thus a thin layer of capacitance will build up between the electrode and the electrolyte sample [44, 45]. The reversal of applied electric field occurs rapidly at high frequency so that most of the ions may stay in the bulk of the sample, which lead to reduction in electrode polarization, and hence decreasing the ɛ’ value [46]. The variation of dielectric constant of PVA with NaTf content at 100 kHz is presented in Fig. 8. It is evident that the dielectric constant increases with increasing NaTf concentration. This can be ascribed to the increase in the number of charge carriers as well as an increase in polarization [24, 47]. These results indicate that dielectric constant can be used to investigate the conductivity of polymer electrolytes.
The dielectric response caused by ion relaxation has been studied using the electric modulus in which the electrode polarization effects are suppressed, i.e. emphasize small features at high frequencies [48]. The real and imaginary parts of electric modulus are related to impedance data through the following equations [36, 49],
Figures 9 and 10 shows the plot of frequency dependance of real (M’) and imaginary (M’’), parts of the electrical modulus, respectively. The plot of real part of modulus spectra (Fig. 9) displays a low value at lower frequencies. This can be ascribed to the large value of capacitance associated with the electrodes that facilitates the migration of ion conduction. It can be seen that the M spectra exhibits dispersion at high frequencies. This confirms the non-Debye behavior in the samples [50]. Conductivity relaxation peaks can be observed in the imaginary part of modulus spectra (see Fig. 10) at low salt concentration. It can be seen that the relaxation peak shifted to higher frequency side with increasing NaTf concentration. This indicates that relaxation time (τo = 1/ωmax) decreases with increasing NaTf salt concentration. The decrease of relaxation time is related to the increase of ionic mobility in amorphous phase of the electrolyte samples. The XRD results strongly support the electrical properties.
Figure 11 shows the Argand plot (M’’ vs. M’) for pure and NaTf-doped PVA samples. It is obvious that Argand plots show an incomplete half semicircle. This reveals the non-Debye type relaxation process [37]. The observed long tail at low frequencies is attributable to the large value of capacitance associated with electrode polarization [49]. The high value of dielectric constant at low frequencies (see Fig. 7) confirms the above interpretation for the long tail of M’ and M’’. It was reported, that if Argand plot has semicircular behavior, then the relaxation is due to conductivity relaxation process, otherwise, it is due to viscoelastic relaxation (or polymer molecular relaxation) [51, 52]. Moreover it can be seen that with increasing salt concentration M″–M′ curve deviates more from the semicircular arc. This can be ascribed to the increase in conductivity [49]. With the increase of NaTf salt concentration the Zi and Zr values are decreased and as it was appeared from impedance plots the high frequency semicircle disappeared (see Fig. 4). Thus the M” and M′ values (\(M''=\omega \,{C_o}{Z_r}\), \(M'=\omega \,{C_o}{Z_i}\)) are more deviate towards the origin.
3 Conclusions
In this work solid polymer electrolytes based on PVA:NaTf bas been prepared by the solution cast technique. The XRD results revealed that the crystalline domain of PVA reduced upon the addition of NaTf salt. The decrease in the diameter of impedance plots is an evidence for the increase of ionic conductivity at high salt concentration. The DC ionic conductivity versus temperature exhibited Arrhenius behavior. The decrease in activation energy with increasing NaTf concentration is interpreted on the bases of amorphous enhancement. The estimated DC conductivity from the AC spectra is comparable with DC conductivity calculated from the bulk resistance (R b ). The high DC conductivity of PVA:NaTf system (present work) compared to other PVA:NaX (X = I, F, Br) was explained on the bases of salts lattice energy. We concluded that the lattice energy of salts significantly affects the DC conductivity of polymer electrolytes. High DC conductivity can be achieved for polymer electrolytes incorporated with low lattice energy salts The Rice and Roth model was used to determine the number density of ions. It was appeared that the increase in DC conductivity attributed to the increase in density of mobile ions as well as their mobility. The results of XRD analysis support the increase of mobility. The dielectric constant increased with increasing salt concentration. The observed long tail in M’ and M’’ spectra are related to the high dielectric constant. The incomplete semicircle of M’–M’’ plots confirms that the relaxation process in the present samples are non-Debye behavior.
References
M. Johnsi, S.A. Suthanthiraraj, Chin. J. Polym. Sci. 34, 332–343 (2016)
S.G. Rathod, R.F. Bhajantri, V. Ravindrachary, B. Poojary, P.K. Pujari, T. Sheela, J. Naik, J. Elastom. Plast. 48, 442–455 (2016)
N. Shukla, A.K. Thakur, A. Shukla, D.T. Marx, Int. J. Electrochem. Sci. 9, 7644–7659 (2014)
M. Muthuvinayagam, C. Gopinathan, Polymer 68, 122–130 (2015)
O.Gh. Abdullah, J. Mater. Sci. 27, 12106–12111 (2016)
Y. M. Yusof, H. A. Illias, M. F. Z. Kadir, Ionics 20, 1235–1245 (2014)
S. F. Bdewi, O. Gh. Abdullah, B.K. Aziz, A.A.R. Mutar, J. Inorg. Organomet. Polym. Mater. 26, 326–334 (2016)
P. B. Bhargav, V. M. Mohan, A. K. Sharma, V. V. R. N. Rao, Ionics 13, 173–178 (2007)
P. B. Bhargav, V. M. Mohan, A. K. Sharma, V. V. R. N. Rao, Ionics 13, 441–446 (2007)
P.B. Bhargav, V.M. Mohan, A.K. Sharma, V.V.R.N. Rao, Curr. Appl. Phys. 9, 165–171 (2009)
E. Sheha, M.K. El-Mansy, J. Power Sources 185, 1509–1513 (2008)
Ch. V. S. Reddy, A.-P. Jin, Q.-Y. Zhu, L.-Q. Mai, W. Chen, Eur. Phys. J. E 19, 471–476 (2006)
S.B. Aziz, Z.H.Z. Abidin, J. Soft Matter 2013, 8 (2013)
O.Gh. Abdullah, S.A. Saleem, J. Electron. Mater. 45, 5910–5920 (2016)
S. B. Aziz, J. Electron. Mater. 45, 736–745 (2016)
O. Gh. Abdullah, S.B. Aziz, M.A. Rasheed, Results Phys. 6, 1103–1108 (2016)
N. Rajeswari, S. Selvasekarapandian, S. Karthikeyan, C. Sanjeeviraja, Y. Iwai, J. Kawamura, Ionics 19, 1105–1113 (2013)
O.Gh. Abdullah, Y.A.K. Salman, S.A. Saleem, J. Mater. Sci. 27, 3591–3598 (2016)
F.K.M. Genova, S. Selvasekarapandian, S. Karthikeyan, N. Vijaya, S. Sivadevi, C. Sanjeeviraja, Polym-Plast. Technol. Eng. 55, 25–35 (2016)
A.S.A. Khiar, A.K. Arof, Ionics 16, 123–129 (2010)
M. Hema, S. Selvasekarapandian, G. Hirankumar, Ionics 13, 483–487 (2007)
S. Badr, E. Sheha, R.M. Bayomi, M.G. El-Shaarawy, Ionics 16, 269–275 (2010)
A.R. Polu, R. Kumar, H.W. Rhee, Ionics 21, 125–132 (2015)
S.B. Aziz, Z.H.Z. Abidin, Mater. Chem. Phys. 144, 280–286 (2014)
S.B. Aziz, Iran Polym. J. 22, 877–883 (2013)
S.B. Aziz, Z.H.Z. Abidin, A.K. Arof, Phys. B. 405, 4429–4433 (2010)
N.S. Salleh, S.B. Aziz, Z. Aspanut, M.F.Z. Kadir, Ionics 22, 2157–2167 (2016)
F. Ciardelli, E. Tsuchida, D. Wöhrle, “Macromolecule-Metal Complexes”. doi:10.1007/978-3-642-60986-2 (Springer, Berlin, 1996)
A. Pawlicka, M. Danczuk, W. Wieczorek, E.Z., Monikowska, J. Phys. Chem. A. 112, 8888–8895 (2008)
G. Polizos, A. Kyritsis, P. Pissis, V.V. Shilov, V.V. Shevchenko, Solid State Ionics 136, 1139–1146 (2000)
A.K. Arof, M.Z. Kufian, M.F. Syukur, M.F. Aziz, A.E. Abdelrahman, S.R. Majid, Electrochim. Acta 74, 39–45 (2012)
V. Aravindan, P. Vickraman, Eur. Polym. J 43, 5121–5127 (2007)
Y. Pavani, M. Ravi, S. Bhavani, A.K. Sharma, V.V.R.N. Rao, Polym. Eng. Sci 52, 1685–1692 (2012)
H.P. de Oliveira, M.V.B. dos Santos, C.G. dos Santos, C.P. de Melo, Mater. Charact. 50, 223–226 (2003)
C.S. Ramya, S. Selvasekarapandian, T. Savitha, G. Hirankumar, R. Baskaran, M.S. Bhuvaneswari, P.C. Angelo, Eur. Polym. J 42, 2672–2677 (2006)
S.B. Aziz, Z.H.Z. Abidin, J. Appl. Polym. Sci. 132, 41774 (2015)
S.B. Aziz, Appl. Phys. A. 122, 706 (2016)
M.J. Rice, W.L. Roth, J. Solid State Chem. 4, 294–310 (1972)
S. R. Majid, A.K. Arof, Phys. B. 355, 78–82 (2005)
N. Sakhavand, P. Muthuramalingam, R. Shahsavari, Langmuir 29, 8154–8163 (2013)
N.A.N. Aziz, N.K. Idris, M.I.N. Isa, Int. J. Polym. Anal. Charact. 15, 319–327 (2010)
J. Siva Kumar, A.R. Subrahmanyam, M. Jaipal Reddy, U.V. Subba Rao, Mater. Lett. 60, 3346–3349 (2006)
S.B. Aziz, Bull. Mater. Sci. 38, 1597–1602 (2015)
S. Navaratnam, K. Ramesh, W.J. Basirun, Mater. Res. Innov. 15, S184–S186 (2011)
S.B. Aziz, Z.H.Z Abidin, A.K. Arof, Expr. Polym. Lett. 4, 300–310 (2010)
M.F. Shukur, R. Ithnin, H.A. Illias, M.F.Z. Kadir, Opt. Mater. 35, 1834–1841 (2013)
M. Singh, V.K. Singh, K. Surana, B. Bhattacharya, P.K. Singh, H.W. Rhee, J. Ind. Eng. Chem. 19, 819–822 (2013)
R. Richert, J. Non-Cryst. Solids 305, 29–39 (2002)
Shujahadeen B., Aziz, Adv. Mater. Sci. Eng. 2016, 11 (2016). doi:10.1155/2016/2527013
R. Baskaran, S. Selvasekarapandian, N. Kuwata, J. Kawamura, Y. Hattori, Mater. Chem. Phys. 98, 55–61 (2006)
S. Ayesh, Chin. J. Polym. Sci. 28, 537–546 (2010)
K. Mohomed, T.G. Gerasimov, F. Moussy, J.P. Harmon, Polymer 46, 3847–3855 (2005)
Acknowledgements
The authors like to thank the Ministry of Higher Education and Scientific Research-Kurdistan Region, University of Sulaimani and University of Human Development, for the financial support given to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aziz, S.B., Abdullah, O.G. & Rasheed, M.A. Structural and electrical characteristics of PVA:NaTf based solid polymer electrolytes: role of lattice energy of salts on electrical DC conductivity. J Mater Sci: Mater Electron 28, 12873–12884 (2017). https://doi.org/10.1007/s10854-017-7117-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7117-x