Abstract
VO2 films were grown on TiO2 (001) substrate by a radio frequency (RF)-plasma assisted oxide molecular beam epitaxy. An excellent reversible metal-to-insulator (MIT) transition accompanied with an abrupt change in both resistivity and infrared transmittance was observed at room temperature (RT), which was much lower than the 341 K for bulk single crystal VO2. Remarkably, the MIT transition temperature (TMIT) deduced from resistivity-temperature curve was well consistent with that obtained from the temperature dependent IR transmittance. The lowed TMIT was supposed to be originated from the internal stress induced by the interface lattice mismatch between VO2 film and TiO2 substrate, this assumption was supported by both Raman measurement and X-ray diffraction (XRD) 2theta peak shift. This achievement will potentially open up new opportunities for advanced applications of VO2-based devices where RT MIT is necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
By virtue of its unique and fascinating properties, VO2 was considered to be one of the key materials for a wide range of energy-related applications [1, 2]. It undergoes an abrupt reversible phase transition, known as metal-to-insulator transition (MIT) or semiconductor-to-metal (SMT) first-order transition. At temperatures below the transition temperature (TMIT), VO2 is in semiconducting state with monoclinic structure (space group P21/c), in which the V atoms pair open an energy gap of 0.6 eV. While at temperatures above TMIT, VO2 is in metallic state with rutile-tetragonal structure (space group P42/mnm), in which overlap between the Fermi level and the V3d band eliminates the band gap [3]. Most notably, this allotropic transition in crystal symmetry and electronic band structure, which can be triggered by some specific external stimuli such as temperature or voltage, was usually accompanied by an abrupt and dramatic change in physical properties. For example, the electrical resistance jumps up to four orders of magnitude and the optical transmission shows a distinct switching effect especially within the infrared wavelength region across the MIT boundary. With these unique and fascinating properties, VO2 was considered to be one of the most promising energy-saving material for a wide range of energy-related applications, including smart windows for energy utilization, supercapacitors for energy storage, and thermoelectric generators for energy conversion. However, the intrinsic TMIT for bulk single crystals VO2 has been well accepted to be ~341 K, the specific applications will be seriously limited by the relatively rigid TMIT [4]. Successfully modulating the TMIT to room temperature(RT) has been a long-standing research topic. For quite a long time, intentionally impurity doping had been commonly attempted to achieve controllable TMIT since both shifts towards lower and higher temperature can be realized by selecting appropriate dopant and ratios, i.e., doping with high-valent metal cations (such as W6+,Nb5+,Mo6+) were reported to lower the TMIT, while doping with low-valent metal cations (such as Al3+, Ga3+ and Cr3+) were expected to increase the TMIT [4]. Nevertheless, the intentionally impurity doping generally suffered from the concomitant deterioration in change magnitude of electrical and optical characteristics as well as broadened hysteresis width, which was certainly undesirable for its practical applications as smart materials. Thus, the most critical challenge for VO2 material lies in finding an effective and convenient way to reduce the TMIT to room temperature while maintaining its abrupt MIT transition.
Recently, there has been a growing interest in modulating MIT behaviors of VO2 films through internal stress since a relatively large TMIT dependence on uniaxial stress along the c axis was proposed theoretically, which might be the most effective way to decrease the TMIT due to its inherent advantages for many practical applications [5, 6]. Following this idea, the MIT process of the MBE grown VO2 films was modulated continuously via the interfacial strain in our recent report [2]. However, there has been little investigation on the intimate correlation between electrical and optical properties across the MIT, which may shed light on the mechanism responsible for the specific MIT behavior in VO2 film, for which no consensus has been reached.
In this study, VO2 films with superior crystalline quality and ideal chemical stoichiometry were grown on TiO2 (001) substrate by oxide molecular beam epitaxy (O-MBE). A systematical analysis were made on the crystal structure, morphology, electrical transport and transmittance within the infrared region (IR) of the VO2 film. The results indicated that the RT MIT performance with narrow hysteresis width and large amplitude contrast in both IR optical transmittance and resistivity was realized, and the TMIT deduced from resistivity–temperature curve was well consistent with the one obtained from the temperature dependent infrared (2 μm) transmittance. Our results will be favorable for exploit the advanced device applications based on VO2 film working near RT [2, 7].
2 Experimental details
VO2 films were grown on single crystal TiO2 (001) substrate by a radio frequency (RF)-plasma assisted MBE with a base pressure better than 5.5 × 10−7 Pa. The details of the epitaxial film preparation have been reported elsewhere [2, 3]. The rutile TiO2 single crystal is selected as substrate due to its stable thermal properties and the same crystal structure and space group as that of VO2 film in high temperature tetragonal metallic phase. In addition, the measurements of electrical and optical properties across the MIT of VO2 will not be affected considering the insulating and transparent characteristics of TiO2 substrate. The TiO2 (001) substrates with the dimensions of 10 × 10 × 0.5 mm3 were thoroughly cleaned to remove surface contamination and provide a atomically smooth surface for epitaxy deposition of VO2 film. All the growth parameters, including the substrate temperature, the chamber pressure, the metallic vanadium evaporation rate and growth duration time, have been optimized for high crystalline quality and abrupt high magnitude MIT property. After deposition, the samples were cooled down to RT under the same oxygen pressure.
The crystallographic properties were determined by X-ray diffraction (XRD) using a LabXRD-6000 (CuKα1:λ = 0.154056 nm). The surface morphology was examined by atomic force microscopy (AFM) with tapping mode using MI PicoScan 2500. The MIT properties were investigated by monitoring the change in both electrical resistivity and infrared transmittance across the MIT process, i.e., the samples were thermally cycled in the temperature ranging from 10 to 45 °C. The resistivity–temperature (R–T) behavior was investigated by measuring the electrical resistivity during heating and cooling process using the conventional electrical method by Keithley 2635 A source meter with 4-probe configuration. The temperature dependent infrared transmittance was studied by detecting the transmittance in the wavelength ranging from1400 to 5000 nm using a FT-IR FTIR SPECTROMETER (Bruker EQUINDX 55), and the transmittance in 2 μm wavelength was recorded as a function of temperature. The films composition and valence state of V were investigated by X-ray photoelectron spectroscopy (XPS) analyses on Thermo Scientific ESCALAB250Xi system with an AlKα 1486.8 eV X-ray radiation source under a base pressure of 3 × 10−8 Pa. Before measurement, the sample was sputtered by Ar ion bombardment for 30 s for surface cleaning. The O1s binding energy line at 530 eV was taken as a reference for calibration and the composition was calculated from the peaks areas taking into account the sensitivity factors. Raman spectra measurements were carried out on DXR Raman Microscope with a 532 nm excitation laser source.
3 Results and discussion
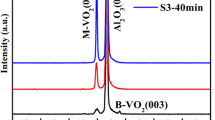
Figure 1a shows the typical XRD patterns of the VO2 film grown on single crystal TiO2 (001) substrate by MBE under optimized conditions. For comparison, the XRD patterns of high quality VO2 film (~60 nm) grown on c-plane sapphire substrate were also present in Fig. 1a, which exhibit MIT properties very close to bulk single crystal VO2 material according to our previous experiments [8]. In addition to the dominant diffraction peaks corresponding to the TiO2 substrate, the well-defined XRD diffraction peak corresponding to the VO2 (002) was observed at 65.6° (JCPDS 76–0675) [9]. It should be noted that the growth of high quality pure phase VO2 film was still rather challenging due to the complexity of the vanadium-oxygen system [10]. Here, however, the high quality pure monoclinic phase VO2 films with (002) preferred orientation have been achieved by MBE based on our previous optimization process [3, 8]. Comparing with the lower XRD profile, the only difference lies in the preferred orientation of VO2 films, which was no doubt resulted from the induction of TiO2 substrate. Because it was very likely that very thin VO2 films (around 30 nm for current case) below a critical thickness are constrained to have the same lattice structure as the tetragonal TiO2 (001) substrate. Here, the critical thickness refers to the thickness at which the misfit dislocations are generated to relax the film and form the equilibrium phase. It has been demonstrated that the phase transformation and strain relaxation phenomena follow totally different kinetics in thin and thick layers [10]. In addition, according to Bragg equation: 2dsinθ = nλ, where the d is crystal plane spacing, θ is incident angle, n and λ are integers and constants, respectively. The slightly shift in VO2 (002) 2θ angle compared with the value for single crystal VO2 material (JCPDS 76-0675), indicating a smaller crystal plane spacing d and thus implying the existence a tensile stress in-plane mainly originated from the misfit between VO2 film and TiO2 substrate. Moreover, the surface morphology of the VO2 film is illustrated by AFM measurements. The AFM images with the scanned area of 1 × 1 µm2 and 2 × 2 µm2 were shown in the Fig. 1b, c, respectively. As can be seen from these images, the sample exhibit smooth and homogeneous surface morphology composed of uniformly and dense grains with spherical granular crystallites, and the same root mean square (rms) was observed to be 0.7 nm for both images. While the uniform and dense nano columns are more clearly visible in the images of Fig. 1c.
a XRD patterns of VO2 film grown on TiO2 (001) substrate by MBE. For comparison, the XRD patterns of high quality VO2 film grown on c-plane sapphire substrate were also present in Fig. 1, which exhibit MIT properties very close to bulk single crystal VO2 material. b and c show the surface morphology AFM images of as-grown VO2 film with the scanning area of (1 × 1 µm2) and (2 × 2 µm2), respectively
The XPS measurements were carried out to identify the valence state of V in the VO2/TiO2 structure (Fig. 2). The XPS survey spectra was shown in Fig. 2a, all the elements can be well identified in the survey spectra according to previous reports in literature [11, 12]. The detected signal from C1s could be attributed to unintentionally contamination from ambient during the growth and measurement process since C element was ubiquitous element in the environment. To identify the precise valence state of V in the oxide layer, the enlarged high-resolution XPS spectra focusing on V2p3/2 and O1s with binding energy between 510 and 533 eV was shown in Fig. 2b. The O1s peak could be de-convoluted into two peaks, only the one at 530.0 eV related to vanadium oxides, whereas the other peak at 527.8 eV comes from titanium oxide. Meanwhile, the typical two-peak structure (V2p3/2 at 515.6 eV and V2p1/2 at 523.3 eV) due to the spin–orbit splitting was observed for the V2p signal. According to Silversmit’s method, the binding energy span between O1s and V2p3/2 core level could be utilized as a criterion to identify the different vanadium oxidation states, since it differs remarkably for VOx with disparate valence state [11]. The calculated binding energy span from Fig. 2b was 14.4 eV, which was in rational agreement with the value of 14.35 eV for stoichiometric VO2 epitaxial layer [13], suggesting that the valence state of V in as-grown VO2 film was principally composed of V4+ [11]. Moreover, the slightly asymmetric feature of the V2p3/2 peak suggests the existence of trace amount of V3+ in the VO2 film. After subtracing the Shirley background (cyan) using Gaussian Lorentzian sum function by XPSPEAK 4.1 software, the V2p3/2 peak could be de-convoluted into two peaks, i.e., V4+ peak at 515.6 eV and V3+ peak at 513.8 eV, no other valence state of V was detected. The oxygen deficiency during high vacuum growth might be responsible for the formation of V3+. It should be noted that the commonly observed V5+ peak at ~517.0 eV, which are usually caused by the surface oxidization as a slighly oxygen rich composition generally exist only on the surfaces (the top several nanometers), was not detected in current case. It has been confirmed that the MIT characteristics might be seriously degraded by the existence of a small amount of vanadium oxides with different stoichiometric composition rather than VO2, such as V2O3 or V2O5, which may exist at the surface or grain boundaries of VO2 grains, although may not be detected by the XRD measurement [14, 15]. So the ideal stoichiometry composition was imperative for reliable material properties of VO2 film. Here, the ratio of oxygen to vanadium can be roughly estimated to be 1.91 from the integrated area of I V 4+ and I V 3+ by the empirical formula: (2I V 4+ + 1.5I V 3+)/(I V 4+ + I V 3+) [14], indicating the nearly stoichiometrical O/V ratio inside the prepared VO2 film. Therefore, the super crystalline quality and ideal chemical stoichiometry of our MBE grown VO2 film were clearly evidenced by XRD, AFM and XPS investigation.
a XPS survey spectra with binding energy in the range of 0-900 eV for VO2/TiO2 structure. b The enlarged high-resolution spectra with binding energy in the range of 510–533 eV and the fitting results for sample VO2/TiO2 heterostructure. After subtracting the Shirley background (cyan curve), the V2p3/2 peak could be de-convoluted into V4+ and V3+ peaks
The MIT performance of the as-grown VO2 sample was investigated by the temperature dependent electrical resistivity and IR optical transmittance measurements across the MIT process. As shown in Fig. 3, an excellent reversible MIT phase transition accompanied with an abrupt change in both electrical resistivity and IR optical transmittance was observed, and the smooth transition profiles suggesting a first-order reversible MIT behavior for the VO2 film grown on TiO2 (001) substrate. The differential d(R)/d(T) versus temperature curves for heating and cooling branches were shown in the left bottom of Fig. 3a, and the TMIT can be determined from the Gaussian fit of the d(R)/dT curves (at which the value reach its extremum). For comparison, the result for the VO2 film grown on c-plane sapphire substrate, which exhibit MIT properties very close to bulk single crystal VO2 material according to our previous report [8], were also shown in the top right corner of Fig. 3a. The MIT properties can be characterized by the following parameters: TMIT (defined as peak temperature for both heating and cooling runs), ΔH (MIT thermal hysteresis width, defined as the difference of TMIT for heating and cooling branch), ΔT (MIT sharpness, characterized by the FWHM of the derivative curve of dR/dT-T plot), as well as ΔA (MIT amplitude, defined as ratio of resistivity in insulator phase to that in metallic phase in the heating process). So it can be seen that the TMIT show remarkable shift towards lower temperature, i.e., 68 °C for previous VO2 film on c-plane sapphire substrate while RT (26 °C) for current VO2 film on TiO2 (001) substrate. While other MIT parameters (including ΔH, ΔT, and ΔA) remains almost on the same orders of magnitude as that in the top right corner of Fig. 3a, suggesting the distinct abrupt MIT performance was maintained perfectly during the decrease of TMIT to RT. The relatively large MIT amplitude of exceeding three orders of magnitude and a narrow hysteresis width will be especially imperative for switching-type applications. Generally extremely demanding processing conditions are required for VO2 films with lowered TMIT and abrupt MIT behavior [8]. So the MIT investigation further confirming the high crystalline quality and phase purity of current VO2 film, which was in well agreement with above XRD, AFM and XPS analysis. Moreover, the IR transmittance at 2 μm wavelength was recorded during heating and cooling process as shown in Fig. 3b, and the differential d(Tr)/d(T) (Tr stands for transmittance) versus temperature curves were shown in the insets of Fig. 3b. The TMIT determined from the Gaussian fit of the d(Tr)/dT curves was 25 °C. Remarkably, the rather small hysteresis width was comparable with the high quality stoichiometric VO2 film grown on c-plane sapphire substrate, which will be especially favorable for rapid response to environmental temperature as thermochromic smart window application. Therefore, the TMIT and hysteresis width deduced from R–T curve (Fig. 3a) was well consistent with the one obtained from the temperature dependent infrared (2 μm) transmittance(Fig. 3b).
a Thermal hysteresis loops of resistance for the VO2 film grown on TiO2(001) substrate by MBE. For comparison, the thermal hysteresis loops for the VO2 film grown on sapphire substrate were shown on the top right corner. The differential d(R)/d(T) versus temperature curves were shown in the corresponding insets. b Thermal hysteresis loops of IR transmission at 2 μm wavelength for VO2 film grown on TiO2 (001) substrate. The differential d(Tr)/d(T) versus temperature curves were shown in the inset to determine the TMIT
According to theoretical prediction, the observed remarkable decrease of TMIT should be closely related with the internal stress induced by the interface lattice mismatch between TiO2 substrate and VO2 film [2]. Herein, the Raman spectra measurements were carried out since the line shape of Raman spectrum was extremely sensitive to the residual stress inside the films. For comparison, the Raman spectra for high quality VO2 film with fully relaxed stress were also present with black line, which exhibit peaks very close to bulk single crystal VO2 material according to our previous experiments [8]. As can be seen from Fig. 4, all the main peaks can be well indexed to the monoclinic VO2, especially the Raman peak at 617 cm−1 (which can be regarded as the signal of M–VO2) was quite pronounced, indicating that the high quality single phase VO2 films were obtained on TiO2 substrate [16]. Moreover, a slight red shift in the main peaks indicating an in-plane tensile stress due to the lattice mismatch, which was also supported by the XRD 2theta peak shift as mentioned above. As expected from Poisson effect (shown in the inset of Fig. 4a, as the VO2 film was stretched in plane, it would tend to be compressed out-of-plane, i.e., in the direction perpendicular to the direction of expansion. Fig. 4b, c show the schematic diagram of microscopic structure of the epitaxy VO2 (002)/TiO2 (001) interface with VO2 in monoclinic (T < TMIT) and tetragonal (T > TMIT) structure. And the lattice parameters of VO2 and TiO2, together with the corresponding lattice mismatch between them were listed in Table 1. Since the lattice parameters of VO2 are smaller than those of TiO2 in both forms, the in-plane tensile stress will be generated for the epitaxially grown VO2 films on TiO2(001) substrate due to the lattice mismatch, further leading to compression along c-axis according to the well-known Poisson’s relation. This was in well consistent with above Raman analysis. Furthermore, as the temperature is lower than the TMIT Fig. 4b, a very large mismatch up to 25% across the VO2 monincial/TiO2 tetragonal interface could be obtained. While as the temperature exceeds the TMIT (Fig. 4c), only a tiny lattice mismatch exist across the VO2 tetragonal/TiO2 tetragonal interface due to the similar tetragonal structure of rutile phase VO2 and TiO2. The reduced V4+–V4+ distance in the crystal structure results in direct overlapping of d orbitals, which stabilized the metallic phase of the rutile structure. In other words, the residual compressive stresses can counteract the transition energy barriers and stimulate the occurrence of the MIT sharply. In addition, the trace amount of V3+ caused by the oxygen deficiency during high vacuum growth might also partially contributes to the decrease of TMIT [2].
a The Raman spectra for VO2 film grown on TiO2(001) substrate by MBE(red line). For comparison, the Raman spectra for high quality VO2 film with fully relaxed stress were also present with black line. b and c show the schematic diagram of microscopic structure of the VO2(002)/TiO2(001) interface with VO2 in monoclinic (T < TSMT) and tetragonal (T > TSMT) structure, respectively. (Color figure online)
4 Conclusion
In summary, the VO2 film with superior crystalline quality and ideal chemical stoichiometry was grown on TiO2 (001) substrates. An excellent RT reversible MIT performance was observed with an abrupt change in both resistivity and infrared optical transmittance. Remarkably, the TMIT deduced from R–T curve was well consistent with the one obtained from the temperature dependent infrared (2 μm) transmittance. Based on the Raman measurement and microstructure model, the RT MIT properties was mainly attributed to the residual compressive stresses imposed by the TiO2 (001) substrates. Our achievement will potentially open new opportunities for advanced applications of VO2-based devices.
References
J.H. Park, J.M. Coy, T.S. Kasirga, C.M. Huang, Z.Y. Fei, S. Hunter, D.H. Cobden, Nature 500, 431 (2013)
L.L. Fan, S. Chen, Z.L. Luo, Q.H. Liu, Y.F. Wu, L. Song, D.X. Ji, P. Wang, W.S. Chu, C. Gao, C.W. Zou, Z.Y. Wu, Nano Lett. 14, 4036 (2014)
M.H. Wang, J.M. Bian, H.J. Sun, H.Z. Liu, X.X. Li, Y.M. Luo, H.L. Huang, Y.Z. Zhang, J. Mater. Sci. 51, 8233 (2016)
S.F. Wang, M.S. Liu, L.B. Kong, Y. Long, X.C. Jiang, A.B. Yu, Prog. Mater. Sci. 81, 1 (2016)
Y. Muraoka, Z. Hiroi, Appl. Phys. Lett. 80, 583 (2002)
K. Martens, N. Aetukuri, J. Jeong, M.G. Samant, S.P. Parkin, Appl. Phys. Lett. 104, 081918 (2014)
K.K. Nagashima, T.S. Yanagida, H.K. Tanaka, Phys. Rev. B 74, 172106 (2006)
J.M. Bian, M.H. Wang, H.J. Sun, H.Z. Liu, X.X. Li, Y.M. Luo, Y.Z. Zhang, J Mater. Sci. 51, 6149 (2016)
M.R. Bayati, R. Molaei, F. Wu, J.D. Budai, Y. Liu, R.J. Narayan, J. Narayan, Acta Mater. 61, 7805 (2013)
C.Z. Wu, F. Feng, Y. Xie, Chem. Soc. Rev 42, 5157 (2013)
Y.X. Guo, Y.F. Liu, C.W. Zou, Z.M. Qi, Y.Y. Wang, Y.Q. Xu, X.L. Wang, F. Zhang, R. Zhou. Appl. Phys. A. 115, 1245 (2014)
K. Zhao, L.T. Teng, Y.F. Tang, X. Chen. Ceram. Int. 40, 15335 (2014)
J. Mendialdua, R. Casanova, Y. Barbaux, J. Electron Spectrosc. Relat. Phenom. 71, 249 (1995)
Y. Zhou, S. Ramanathan, J. Appl. Phys 112, 074114 (2012)
Z. Yang, C. Ko, S. Ramanathan, J. Appl. Phys. 108, 073708 (2010)
F.H. Chen, L.L. Fan, S. Chen, G.M. Liao, Y.L. Chen, P. Wu, L. Song, C.W. Zou, Z.Y. Wu, ACS Appl. Mater. Interfaces 7, 6875 (2015)
Acknowledgements
We acknowledge support from the Fundamental Research Funds for the Central Universities (DUT16LAB11), NSFC (61520106013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest to this work.
Rights and permissions
About this article
Cite this article
Wang, M., Fan, L., Bian, J. et al. Room-temperature metal–insulator transition of MBE grown VO2 film investigated by temperature dependent resistance and transmittance. J Mater Sci: Mater Electron 28, 11046–11052 (2017). https://doi.org/10.1007/s10854-017-6888-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6888-4