Abstract
The effects of CaO–La2O3 additives on sintering temperature and microwave dielectric properties of (Zr0.8Sn0.2)TiO4 (ZST) ceramics prepared by solid-state reaction method were investigated. Structural characterization and microstructure of materials were studied via X-ray diffraction and scanning electron microscopy. crystalline and single phase exhibiting orthorhombic symmetry were identified. Small quantities of CaO and La2O3 promoted the densification of the ceramics improving dielectric properties, otherwise, an excessive CaO and La2O3 amount gave an abnormal grain growth which deteriorated dielectrical parameters. Dense ZST ceramics doped with 0.5% CaO and 1 wt% La2O3 were attained after sintering at 1335 °C during 4h, in air atmosphere. Microwave dielectric parameters ofε r = 39.56, Q × f = 44100 GHz (at 5.6 GHz), τ f = −1.66 ppm/°C were derived.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microwave dielectric ceramic material is a crucial component in many wireless communication technologies such as: resonators, direct broadcasting satellites, intelligent transport systems, and wireless local area networks (WLAN). To meet the demand for smaller, high-performing microwave devices, it is necessary to develop middle dielectric and low-loss microwave dielectric materials [1–4].
(Zr0.8Sn0.2)TiO4 (ZST) satisfies many dielectric resonator requirements at microwave frequencies due its quality factor (Q > 5000) which allows rapid electronic signal transition at ultra-high frequencies, high dielectric constant (ε r > 30) for possible size miniaturization, and near-zero temperature coefficient of resonant frequency (τ f < ±10 ppm/°C) for temperature stable circuits. The host structure of ZST system is ZrTiO4, which has a α-PbO2-type crystal structure belonging to the Pbcn space group. Both Ti and Zr cations are ordered in the low-temperature phase and disordered in the high-temperature phase. A temperature-stable ZST can be obtained by replacing 20 mol% Zr ions with Sn ions [5–7], but even then, ZST ceramics are disadvantaged by low sinterability. Properly densified ZST ceramics can only be prepared with the use of a sintering aid. Besides, their low Q × f values also make them very impractical in communication technology because the Q × f would be dramatically worsened by adding additives for ZST ceramics. Fortunately, many researchers have attempted to improve the sinterability and maintain the Q × f values of ZST ceramics through the use of various dopants (e.g., La2O3, BaO, CaO and V2O5) [8–11].
Kim et al. for example, found that the addition of CaO does not affect the dielectric constants (ε r ) or temperature coefficients of resonant frequency (τ f ) while improving the quality of ZST ceramics [9]. Zhang et al. reported that with 1.0 wt% La2O3 and 2.0 wt% BaO additives, ZST ceramics sintered at 1350 °C exhibited comprehensive performance of ρ ~ 5.10 g/cm3, ε r ~ 41, Q ~ 9800 and τ f ~ −3.79 ppm/°C [11]. That being said, both CaO and La2O3 are classical additives to enhancement of sinterability and phase stabilization in ZST ceramics.
The present work reports the influence of CaO (0.5–2.0 wt%) and La2O3 (1.0 wt%) additives on the phase composition, microstructures, sinterability, and microwave dielectric properties of ZST ceramics, to show the excellent temperature stability of the resonant frequency.
2 Experimental
2.1 Ceramics preparation
Samples of ZST ceramics were prepared by conventional solid-state methods from individual high-purity oxide powders: ZrO2 (99.9%, Handan Saint-Gobain Co., Ltd., Hebei, China, the average particle size ca. 0.3 μm), SnO2 (99.9%, Tin Co., Ltd., Yunnan, China, the average particle size ca. 0.523 μm), TiO2 (99.9%, Yuejiang Titannium Chemical Manufacturer Co., Ltd., Shanghai, China, the average particle size ca. 0.556 μm) and La2O3 (99.9%, Jining Zhongkai New Type Material Science Co., Ltd., Shandong, China, the average particle size ca. 2.316 μm). To obtain CaO, CaCO3 (99.9%, Changzhou Calcium Carbonate Co., Ltd., Jiangsu, China, the average particle size ca. 6.313 μm) powders were calcined at 1200 °C for 6 h. The starting materials were mixed according to the desired stoichiometry of (Zr0.8Sn0.2)TiO4 ceramics. The powders were ground in distilled water for 8 h in a ball mill with zirconia balls. All mixtures were dried, forced through a 100-mesh sieve, and then calcined at 1100 °C for 3 h. The calcined powders were mixed as desired composition with various amounts of CaO and 1.0 wt% La2O3 as sintering aids and re-milled with zirconia balls for 8 h in distilled water and dried. The dried powders with 8 wt% of a 5% solution of PVA as a binder were screened by a 100-mesh. The final powders were pressed uniaxially under 150 MPa into disk pellets with a diameter of 11 mm and thickness of 5 mm. The pellets were sintered at various temperatures from 1295 to 1375 °C during 4 h, in air atmosphere.
2.2 Ceramics characterization
The apparent densities of the sintered specimens were measured by Archimedes method. The crystalline structure analysis of ZST ceramics was identified by X-ray diffractometer (XRD; RIGAKU; Smartlab 3) using Ni filtered Cu Ka radiation (λ = 0.1542 nm, 40 kV, 20 mA, 2θ = 20°–70°). Its morphology and grain size were examined by field emission scanning electron microscopy (Model JEOL JSM-7600F, FEI Co., Japan). The fractured surfaces of the sintered ceramics were ground (to 1200 grade SiC) and polished (to 1 μm diamond paste) and then thermally etched at 1350 °C for 60 min and coated with Au prior to SEM analysis. The average grain size was determined by measuring the mean linear intercept of the grains as described by a book “Standard test methods for determining average grain size” [12]. The microwave dielectric properties of sintered specimen were measured by a network analyzer (N5234A, Agilent Co, America) in the frequency range of 5–6 GHz. The dielectric constants were measured by exciting the TE01 resonant mode of dielectric resonator as suggested by Hakki-Coleman [13]. The unload quality factors were measured using TE01 mode by the cavity method [14]. Temperature coefficients of resonant frequency were measured in the temperature range of 25–80 °C. The τ f (ppm/°C) measured at 5.6 GHz was calculated by equation:
where \({f_{80}}\) was the resonant frequency at 80°Cand \({f_{25}}\) was the resonant frequency at 25 °C.
3 Results and discussion
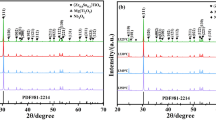
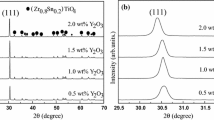
The XRD patterns of ZST ceramics with 0–2.0 wt% CaO and 1.0 wt% La2O3 sintered at 1335 °C for 4 h are shown in Fig. 1. Only a single (Zr0.8Sn0.2)TiO4 (orthorhombic structure, space group α-PbO2, JCPDS Card No. 81-2214) phase can be observed. It is accepted that La2O3 forms a secondary phase at the grain boundary, but there was no such minor phase in our XRD patterns because it is difficult to observe minor secondary phases using XRD [15]. In effect, doping of ZST ceramics with small amount of CaO and La2O3 did not influence the phase composition of this system. Figure 1(b) shows the dominant diffraction lines (111) of ZST ceramics, where the peaks shift to smaller angles of 2 theta as CaO content increases. According to the Bragg equation, crystalline interplanar distance is inversely proportional to sin(theta) [16]. The unit cell constants a0, b0, c0, and unit cell volume values were calculated as listed in Table 1. The unit cell volume of ZST ceramics increased to a maximum at 2.0 wt% CaO addition due to the fact that the ionic radii of Ca2+ (1.00 Å) and La3+ (1.032 Å) are larger than that of Zr4+ (0.72 Å), Sn4+ (0.69 Å), or Ti4+ (0.605 Å). As content of CaO increased, the lattice parameter b0 and c0 increased slightly, while lattice parameter a0 and unit cell volume increased greatly. The interplanar distance is directly proportional to the lattice parameter a0, b0 and c0. Therefore, increasing CaO and La2O3 additions appeared to enlarge the interplanar distance and ultimately make the unit cell expand, confirming the results of XRD.
Figure 2 shows a set of fracture surface SEM micrographs of ZST ceramics containing 1.0 wt.% of La2O3 and doped with several amount of CaO sintered at optimum temperature, 1335 °C, during 4h in air atmosphere. The microstructures of the sintered ceramics shown in Fig. 2 indicate well packed grains. As shown in Fig. 2a, b, all samples show a homogenous microstructure and the grain size is generally in the range 5–15 μm. However, with the content of CaO increases from 1.0 wt% to 1.5 wt%, the microstructure morphology of the picture there are some large grain size. When the content of CaO reaches to 2.0 wt%, excessive CaO addition seemed to cause a decreasing of uniformity degree and increasing the sample’s porosity. Grain size and porosity variation with respect to the sintering aid content is critical for understanding the densification and dielectric properties, particularly for dielectric loss [17]. The relation between the grain size and the additive content is shown in Fig. 3. At smaller additive weight percent, the grain size increases rapidly with an increase in sintering aids. As an example, the average grain size for ceramics with 0.5 wt% CaO and 1.0 wt% La2O3, 1.0 wt% CaO and 1.0 wt% La2O3 is 5 and 8 μm, respectively. And that for ceramics with 1.5 wt% CaO and 1.0 wt% La2O3 additives is 13 μm. In addition, at larger (2.0 wt% CaO and 1.0 wt% La2O3) doping amount, some distortion of grains is observed as function of doping magnitude. In this way, the extent of CaO and La2O3 doping play an important role in decreasing of the sintering temperature due to the significant effect of the liquid-phase sintering mechanism [18]. As a function of fraction of CaO selected 2.0 wt%, trapped pores are formed due to inhomogeneous crystal grain size distribution and liquid-phase formation, which provide a great mobility of grains boundaries. In a broad sense, the proper amount of sintering agents benefits the densification and microwave dielectric properties of ZST ceramics [19, 20].
The curves of apparent densities (ρ) of 1.0 wt% La2O3-doped ZST ceramics with different amounts of CaO versus sintering temperature are shown in Fig. 4. Point by point, all densities are inversely proportional to the fraction of CaO. Densities increase as a function of sintering temperature up to a maximum, at 1335 °C, exhibiting a slight decreasing at temperatures higher than 1335 °C. For ZST ceramics with 0.5 wt% CaO, the densities increased from 4.75 to 5.06 g/cm3 as temperature increased between 1295 and 1375 °C, which was about 97.50% of the nominal theoretical density (5.19 g/cm3 for ZST). These results are in accordance with results and discussion of Fig. 2a, b, these demonstrate that dense homogenous microstructures eliminated the pores and increased the apparent densities of ZST ceramics used in this study. Doping with small amount of CaO and La2O3 decreases the sintering temperature and improves the apparent densities of ZST, but sintering aids in excess the apparent density due porosity, see discussion of Fig. 2(c–d).
The dielectric constants (ε r) of 1.0 wt% La2O3-doped ZST ceramics as a function of sintering temperature at different CaO concentrations from 0.5 to 2.0 wt% are shown in Fig. 5. The highest ε r value, 38.90, was obtained for 0.5 wt% CaO and 1.0 wt% La2O3 added specimen sintered at 1335 °C. The variation tendency of dielectric constants versus sintering temperature corresponds to that of apparent densities versus sintering temperature. The variations in ε r observed here can be attributed to several factors. Firstly, the relative densities: more dense samples had more dipoles per unit volume and thus were more liable to be polarized and exhibit higher ε r values [21, 22]. Secondly, it is commonly known that dielectric constants vary with the total molecular dielectric polarizability and molar volume according to the Clausius–Mossotti equation [23], the ionic polarizabilities of Ca2+ (3.17 Å3) and La3+ (6.07 Å3) are larger than that of Sn4+ (2.83 Å3) and Ti4+ (2.93 Å3) ions [24], so the dielectric constants of CaO and La2O3-doped ZST ceramics were larger than that of pure ZST ceramic material [25]. In addition, the expansion of the unit cell volume (Table 1) caused the dielectric polarizability in the unit cell volume to decrease, however, resulting in a decrease of ε r with increasing CaO content (Fig. 6).
Figure 5 shows the Q × f values of 1.0 wt% La2O3-doped ZST ceramics with varying CaO content plotted as a function of sintering temperature, where said Q × f values of all samples increase with sintering temperature from 1295 to 1335 °C, then decrease as temperature continues to increase. The sample with 0.5 wt% CaO and 1.0 wt% La2O3 sintered at 1335 °C for 4 h had the highest Q × f value, 44,100 GHz (at 5.6 GHz). Several factors are known to contribute to dielectric loss in the range of microwave frequencies; said factors can be divided into two categories: intrinsic loss and extrinsic loss. Intrinsic loss is mainly caused by lattice vibration modes while extrinsic loss is dominated by second phases, oxygen vacancies, grain morphology, and densification or porosity [26, 27]. Considering the phase composition, secondary phases can be ignored in the present case. The changes in Q × f values we measured were, as mentioned above, in agreement with the changes in apparent densities. Therefore, the Q × f values of our samples can be attributed to the pellet densification. Interestingly, the grain morphology of the 0.5 wt% CaO and 1.0 wt% La2O3 doped sample was denser than those of the other three samples and it yielded a higher Q × f value. Dense homogenous microstructures indicate reduced lattice imperfections [28], so the decrease in Q × f value could be attributed to the excessive sintering aid (1.5–2.0 wt% CaO content) having caused a normal and/or abnormal grain growth.
A comparison between the properties of undoped and doped ZST ceramics along with their sintering conditions, apparent densities, and microwave dielectric properties is provided in Table 2. The τ f values of 1.0 wt% La2O3 doped ZST ceramics with 0.5, 1.0, 1.5, and 2.0 wt% CaO addition were −1.66, −2.68, −1.23, and −2.41 ppm/°C, respectively. The temperature coefficient of resonant frequency (τ f ) is known to be related to the composition and secondary phases of the materials [29]. Because ZST ceramics are temperature-stable and the additions of CaO and La2O3 did not appear to cause any noticeable secondary phases, the (τ f ) values reported above are relatively stable.
4 Conclusions
The microwave dielectric properties of ZST ceramics were investigated in this study. XRD results showed that CaO and La2O3 doped ZST ceramics are single phase, with an orthorhombic structure (space group: Pbnm). The unit cell volumes of ZST ceramics increase as CaO content increases over the range we investigated. The addition of 0.5 wt% CaO and 1.0% La2O3 could not only efficiently lower the sintering temperature to 1335 °C, but also significant improvement of the densification degree and microwave dielectric properties of ZST ceramics. Furthermore, these microwave dielectric properties appear to be related to CaO and La2O3 doping content, grain morphology, and densification. The excellent microwave properties, the Q × f value of ~ 44,100 GHz (at 5.6 GHz), the ε r value of ~39.56, and the τ f value of ~−1.66 ppm/°C, are obtained for ZST ceramics with 0.5 wt% CaO and 1.0 wt% La2O3 sintered at 1335 °C.
References
S. Peng, J.M. Xu, H. Li, J. Mater. Sci. 26(11), 8819–8823 (2015)
Y.C. Chen, R.Y. Syu, J. Mater. Sci. 27(7), 6979–6984 (2016)
H.T. Wu, Q.J. Mei, C.F. Xing, J. Alloy. Compd 679, 26–31 (2016)
R. Laishram, O.P. Thakur, J. Mater. Sci. 24(9), 3504–3507 (2016)
L.Z. Wang, L.X. Wang, Z.F. Wang, J. Mater. Sci. 27(4), 3929–3933 (2016)
D. Pamu, G.L.N. Rao, K.C.J. Raju, J. Am. Ceram. Soc. 95(1), 126–132 (2012)
S.M. Olhero, A. Kaushal, RSC Adv. 4(89) 48734–48740 (2014)
L. Qian, H.Q. Zhou, Q.X. Jiang, J. Mater. Sci. 27(6), 6183–6187 (2016)
D.J. Kim, J.W. Hahn, G.P. Han, J. Am. Ceram. Soc. 83(4), 1010–1012 (2000)
C.L. Huang, M.H. Weng, C.C. Wu, Jpn. J. Appl. Phys. 40(2A), 698–702 (2001)
S.X. Zhang, J.B. Li, H.Z. Zhai, Ceram. Int. 28(4), 407–411 (2002)
Standard test methods for determining average grain size, American Society for Testing and Materials, 26, E 112–10 (2010)
Y.G. Zhao, P. Zhang, RSC. Adv. 5(118), 97746–97754 (2015)
P. Zhang, Y.G. Zhao, L.X. Li, Phys. Chem. Chem. Phys. 17(26), 16692–16698 (2015)
Q.L. Sun, H.Q. Zhou, X.F. Luo, Ceram. Int. 42(10), 12306–12311 (2016)
W.T. Xie, H.Q. Zhou, H.K. Zhu, J. Mater. Sci. 26(6), 3515–3520 (2015)
R. Kudesia, A.E. McHale, R.L. Snyder, J. Am. Ceram. Soc. 77(12), 3215–3220 (1994)
L.S. Hu, H.Q. Zhou, Q.L. Sun, J. Mater. Sci. 27(12), 12834–12839 (2016)
L.Z. Wang, L.X. Wang, Z.F. Wang, J. Mater. Sci. 26(11), 9026–9030 (2015)
Q.L. Sun, H.Q. Zhou, H.K. Zhu, J. Mater. Sci. 27(8), 7750–7754 (2016)
A. Ullah, Y. Iqbal, T. Mahmood, Ceram. Int. 41(10), 15089–15096 (2015)
Q.X. Jiang, W.T. Xie, Q.L. Cao, J. Mater. Sci. 27(7), 7674–7679 (2016)
X.S. Lyu, L.X. Li, H. Sun, S. Zhang, S. Li, High-Q microwave dielectrics in wolframite magnesium zirconium tantalate ceramics. Ceram. Int. 42(1) 2036–2040 (2016)
R.D. Shannon, J. Appl. Phys 73(1), 348–366 (1993)
S.X. Zhang, J.B. Li, H.Z. Zhai, Mater. Chem. Phys 77(2), 470–475 (2002)
S.D. Ramarao, V.R.K. Murthy, Dalton Trans. 44, 2311–2324 (2015)
L.X. Li, H. Sun, H.C. Cai, J. Alloys. Compd. 639, 516–519 (2015)
C.L. Huang, M.W. Weng, Mater. Res. Bull. 35(11), 1881–1888 (2000)
J.X. Bi, C.F. Xing, X.S. Jiang, J. Mater. Sci. 27(8), 8055–8061 (2016)
A. Ioachim, M.G. Banciu, M.I. Toacsen, Appl. Surf. Sci. 253(1), 335–338 (2006)
Acknowledgements
This work is supported by National Natural Science Foundation of China (No.51578327).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, B., Han, L., Li, B. et al. Effects of CaO and La2O3 doping of (Zr0.8Sn0.2)TiO4 ceramics on the densifying behavior and microwave dielectric properties. J Mater Sci: Mater Electron 28, 9542–9547 (2017). https://doi.org/10.1007/s10854-017-6701-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6701-4