Abstract
To investigate how addition of La2O3 and MgO influenced the structures, phase composition, sintering characteristics, and microwave dielectric properties of (Zr0.8Sn0.2)TiO4 (ZST) ceramics, we synthesized the ceramic samples using solid-state methodology. X-ray diffraction analysis indicated that the doped ZST samples were homogeneous, composed of a single phase with orthorhombic structure. Appropriate content of MgO doping favored grain growth and densification, affected the grain size distribution, and improved the dielectric properties of sintered ZST ceramics. With the sequentially increased in La2O3 and MgO contents, a defective ceramic including incomplete growth of grains, the incompact structures and the increasing porosities was formed. To demonstrate excellent microwave dielectric properties, 0.5 wt% La2O3 and 1.0 wt% MgO were added to ZST ceramics and sintered at 1320 °C for 5 h. Resulting sintered material had a moderate relative permitivitty (εr = 38.44), a superior Q × f value (52670 GHz at 5.6 GHz), and almost zero value for temperature coefficient of resonance frequency (τf = 0.81 ppm/°C).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the ongoing revolution of 4G/5G microwave-based communication, direct broadcasting satellite and microelectronic technologies, the requirement of multifunctional integration and high power miniaturization of microwave devices have broadly increased [1,2,3]. Especially, microwave dielectric ceramics have gained extensive attraction over the past decade due to the rapid development of wireless communication technology. The demand of technology progress provides a tremendous opportunity with (Zr0.8Sn0.2)TiO4 (ZST) ceramics. To be specific, for qualify as a commercially viable candidate as microwave devices, ceramics should have a suitable relative permittivity (εr typically > 25) to largen signal velocity; a higher Q value to improve the transmission quality (Q typically > 3000) for decreasing the signal propagation delay time and suppress signal damping; a near-zero temperature coefficient (τf ~ 0 ppm/°C) for temperature stability of the frequency response across temperature changes [4]. However, the raw materials, the dopants, and the microstructures play a significant role in above-mentioned properties of ZST ceramics [5,6,7,8]. It is almost impossible to prepare complete densified ZST ceramics without any sintering aid. Plenty of scientists have put energy into the role of dopants (e. g., Ba3(VO4)2, MgO and SrO) and their influence on microwave dielectric loss in ZST ceramics [9,10,11]. Recently, Zhang et al. [9] investigated that adding 0.5 wt% Ba3(VO4)2 addition in ZST ceramics showed good microwave dielectric properties of εr = 36.6, Q × f = 46,000 GHz, τf = 1.47 ppm/°C with 1275 °C sintering temperature. The microwave dielectric properties of ZST ceramics doped with 0.2 wt% MgO, 0.6 wt% SrO and 1.0 wt% La2O3 at 1300 °C (εr ~ 40.11, Q × f ~ 51,000 GHz and τf ~ − 2.85 ppm/°C) have been reported by Sun et al. [10]. Qian et al. [11] synthesized ZST ceramics by adding 1.0 wt% La2O3, 0.6 wt% MgO and 0.4 wt% BaO to form material with comprehensive performance of εr of 38.68, a Q × f value of 42,300 GHz and a τf value of + 5.4 ppm/°C when sintered at 1340 °C.
Kim et al. [12, 13] published that a large number of the alkaline earth metal oxides (e.g., CaO, MgO, SrO or BaO) and TiO2 formed eutectic liquids and [TiO4] tetrahedron at sintering temperature below 1400 °C, as a result, these oxides availably reduced fabricating cost of ZST ceramics. Previous studies showed that the addition of La3+ ions influenced the dielectric properties of ZST materials [14]. Ioachim et al. [15] elaborated that the MgO doped ZST samples showed an extremely tiny decrease of the dielectric constant from 1 kHz to 6.5 GHz of less than 1%. However, there was also an exceptional thermal stability of the dielectric constant, in the temperature range − 150–150 °C of 6–7 ppm/°C. Nevertheless, La3+ (added as La2O3) in combination with divalent Mg ion (MgO) isn’t tested as binary dopants in ZST ceramics. This work aimed to thoroughly examine how addition of lanthane (III)-oxide and magnesium oxide influenced structures, phase composition, sintering characteristics, and microwave dielectric properties of ZST materials. The goal was to find the optimal contents of dopants that yielded sintered material with the excellent temperature coefficient of the frequency of resonance.
2 Experimental procedure
ZST ceramics were synthesized by conventional solid state reaction route. The starting powders used in this study were ZrO2 (99.9%, Jinkun Co., Ltd., Zhejiang, China), SnO (99.9%, Haozhao Chemical Co., Ltd. Guangdong, China), TiO2 (99.9%, Yuxing Chemical Co. Ltd., Shandong, China), La2O3 (99.9%, Ganzhou Kemingrui Non-ferrous Materials Co., Ltd. Jiangxi, China) and MgO (99.9%, Weifang Xuhui New Materials Co. Ltd., Shandong, China). The weight percent of La2O3 and MgO were tabulated in Table 1. The ratio of La2O3 and MgO was kept as 1 to 2 in all samples. The various kinds of raw materials were weighed according to their stoichiometric and mixed with deionized water in Si3N4 bottles. After that, the mixed slurries were slowly dried in water bath kettle at 60 °C and then calcined at different temperature (950 °C, 1050 °C and 1150 °C) for 3 h in air atmosphere. After breaking and remilling monolithic calcined block, they were sieved through 60 mesh, the fine powders together with the 5 wt% polyvinyl alcohol were uniaxially compacted into a cylinder mould of 13.1 mm in diameter and 7.0 mm in thickness at 150 MPa pressure. To remove the polyvinyl alcohol, we adjusted the heating program at 450 °C for 5 h and then sintered in the temperature range of 1280–1360 °C for 5 h in air after putting the green bodies into high temperature sintering furnace. The rate of rise of temperature was 1 °C/min.
The relative density and crystal structure of sintered ceramics were determined by the Archimedes method and X-ray diffraction equipment (XRD; RIGAKU; Smartlab 3) with CuKα radiation from 10°–80° range in 2θ, respectively. The microstructures of the sintered material were visualized by scanning electron microscope (Quanta 200 FEG, FEI Co., USA). A network analyzer (N5234A, Agilent Co., Ltd., USA) was evaluated the microwave dielectric properties of the samples. The relative permittivities were measured using the Hakki–Colemanpost–resonator method [16] by exciting the TE011 resonant mode of the DR using the electric probe of an antenna as suggested by Courtney [17]. The TE011 mode of the cavity method was used to determine the unloaded quality factors [18]. All measurements were made in the frequency range of 2–8 GHz at room temperature. The τf values of the TE011 mode were acquired from 25 °C to 80 °C. The τf values were calculated as:
where f80 and f25 figure as the resonant frequencies at corresponding temperatures.
3 Results and discussion
3.1 X-ray diffraction analysis
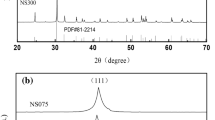
Figure 1 illustrated the XRD patterns of pure ZrO2, SnO2 and TiO2 mixed powders sintered at various calcination temperature for 3 h. Pattern (a) showed the XRD profile of the compacted powders calcined at 950 °C, a orthorhombic structured ZST phase was predominantly identified (JCPDS Card No. 81-2214) by the circles, however, peaks corresponding to small contents of unreacted ZrO2, SnO2 and TiO2 phases were also observed in the profile of the pattern (a). From the pattern (b) and (c), when the compacted powers calcined at 1050 °C or 1150 °C, it could be found that the intensity of ZST phase diffraction peaks moved to a larger angle and decreased apparently without formation of unreacted raw materials phase, indicating that orthorhombic structure increased continuously and pure ZST ceramics would be obtained. All the diffraction peaks in pattern (c) resembled in pattern (b). Wang et al. [19, 20] reported that the relative higher calcined temperature was benefit for the reaction of the main ZST phase. Thus, in order to obtain pure orthorhombic structure, the calcination temperature of ZrO2, SnO2 and TiO2 mixed powders were employed to 1150 °C for 3 h in air in the subsequent experiments.
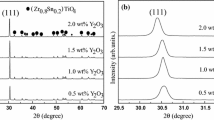
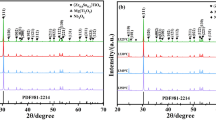
Figure 2 presented the powder XRD patterns of ZST ceramics sintered at 1320 °C prepared with different levels of La2O3 and MgO. From the left-hand side figure, it was evident that all the samples prepared at 1320 °C had extremely similar XRD patterns, with a splitting of diffraction peaks at 2θ ≈ 32.7°. We could not find any impurity peaks in XRD patterns, proving that the sintered ZST ceramics not contained redundant impurity phase. Observed splitting of diffraction peaks corresponded to (111) planes of homogeneous ZST phase, with the space group Pbcn standard JCPDS, file No. 81-2214 of orthorhombic structure. The doping mechanism of ZST ceramics was also investigated. As can be seen from the magnified (111) peaks on right-hand side, the (111) Bragg peaks showed slightly reduced 2θ angles with the increasing La2O3 and MgO contents. To calculate the lattice parameters of the unit cell, we used Rietveld method to refine the diffractograms of ZST ceramics. All parameters listed in Table 2 were calculated accordingly. The continuous increase in lattice parameters was observed. The unit cell volume of LM4 was 0.14454 nm3, while unit cell volumes of LM1, LM2 and LM3 were 0.12768 nm3, 0.12792 nm3 and 0.14254 nm3, respectively. According to the ionic radius tolerance principle, this phenomenon can be explained by the larger volume of La3+ ion (0.1172 nm) compared to Zr4+ (0.072 nm), Sn4+ (0.069 nm) and Ti4+ (0.0605 nm). The increased contents of La2O3 and MgO led to the larger unit cell volumes, which was consistent with the lower shift of diffraction peaks according to the Bragg’s equation.
3.2 Scanning electron micrograph
Typical SEM micrographs of the fractured surfaces of ZST ceramics with different contents of La2O3 and MgO sintered at 1320 °C for 5 h were depicted in Fig. 3a–d. The white points in Fig. 3d were caused by the metal spraying process. Well densified and homogenous ceramics were observed in LM1 and LM2 samples, suggesting the high densification when sintered at optimum temperature. With the increased of La2O3 and MgO contents, the small grains gradually grew up and the size distribution of crystalline grains became more uniform (Fig. 4b). In addition, Fig. 4b showed a low-porous, dense material, suggesting that the minuscule contents of dopants (La2O3 and MgO) favored the densification of ZST ceramics. Further addition of La2O3 and MgO produced an excessive grain growth. As indicated in Fig. 3c and d, LM3 and LM4 samples sintered at 1320 °C showed visible pores and incomplete microstructure, resulting in an insufficient densification. Furthermore, as can be notably seen in Fig. 3b and c, LM2 sample compared with LM3 sample, clearly exhibited legible grain boundaries, however, some of the LM3 sample grains were distorted, and such grains in Fig. 3a and b were not found. Excessive content of MgO might lead to local heterogeneity in composition and liquid phase formation at certain, microscopic parts of ceramics [21]. Besides, the abnormal grain growth might impair the dielectric characteristics of ZST ceramics.
3.3 Sintering characteristics
Figure 4 showed the temperature change in bulk densities (ρ) of ZST materials doped with different contents of La2O3 and MgO. The sintering of ZST ceramics was a densification process [22]. For all ceramic samples, the increase in temperature led to densification of a material, with the maximum density reached at 1320 °C. For higher temperatures, the bulk densities decreased due to over-sintering. When 0.25 wt% La2O3 and 0.5 wt% MgO were added, the bulk densities were relatively low, and the densities increased with the La2O3 and MgO contents increasing.
The maximum bulk density of LM2 sample was 5.08 g/cm3, reaching 97.88% of the theoretical value for pure ZST (5.19 g/cm3). Additionally, for LM3 and LM4 samples, increased contents of La2O3 and MgO decreased the bulk densities, probably owing to the production of liquid phase and subsequent formation of more porous grains (Fig. 3c–d). The degradation of the bulk densities was mainly due to formation of liquid phase as a consequence of the increased content of MgO. This was in accordance with previous studies of Pamu et al. [23].
3.4 Microwave dielectric properties
Figure 5 showed the temperature variation of relative permittivities (εr) of ZST materials, doped with increasing percentages of La2O3 and MgO. With the sintering temperature ranged from 1280–1360 °C, the εr values initially increased, reached a maximum at 1320 °C followed by the decreased at higher temperatures. The εr values varied from 28.98 to 38.44; and a maximum of 38.44 for LM2 sample achieved at 1320 °C. The variation in relative permittivity followed the temperature trend of bulk density (Fig. 4).
Many intrinsic and extrinsic factors might influence εr values [5, 24,25,26,27]. In this report, the εr values of ZST ceramics obtained at different sintering temperatures varied only with bulk densities, as the materials were homogeneous, without the structural defects and formation of secondary structures. Additionally, increased MgO content led to decrease in the relative permittivities of ZST ceramics. The likely reason for this effect was the excess liquid phase formation in the ceramics. Regarding the intrinsic factors, the theoretical value for relative permittivity can be estimated from Clausius–Mossotti equation [28]:
where VM was unit cell volume and x was cell polarizability of ceramics. Unit cell polarizability was related to the expansion characteristics of ZST materials. The more voluminous ions with larger values for dielectric polarizability increased the cell polarizability of a material. Compared to normal constituents of ZST ceramics, La3+ was larger and also more polarizable (dielectric polarizability of La3+ was 6.07 Å3vs. 3.25, 2.83 and 2.93 Å3 for Zr4+, Sn4+ and Ti4+, respectively) [29], which resulted in the increasing of the relative permittivity. The complete list of properties of ZST ceramics (Table 3) confirmed this observation.
Figure 6 demonstrated the temperature changes in Q × f product of ZST materials, doped with various percentages of La2O3 and MgO. The Q × f product varied with the reaction temperature similarly to the apparent bulk density (Fig. 4). More specifically, the Q × f values for all materials firstly rose up to the maximum values at 1320 °C, followed by the decreased at higher temperatures. At 1320 °C, LM2 sample reached a maximum product of 52,670 GHz (at 5.6 GHz). At the same temperature, Q × f values of samples LM3 and LM4 were significantly lower (34,780 GHz and 31,280 GHz at 5.6 GHz, respectively).
The quality of ceramics was determined by the intrinsic and extrinsic losses in dielectric properties. Internal vibrations of the constituents of a material (atoms, ions, electrons) caused intrinsic loss, while the porosity, grain size, crystal defects, phase composition and phase transition yielded extrinsic losses in ceramics [31, 32]. As Q × f values varied with the temperature similarly to the bulk densities, it can be concluded that the densification decreased dielectric loss of sintered ZST materials.
The content of MgO added also influenced the dielectric properties of this material. The surplus MgO (1.5 wt% and 2.0 wt%) induced inhomogeneity in the microstructure of a material and decreased its overall quality. The findings of Pamu et al. confirmed the crucial role of MgO content optimization for obtaining the optimal dielectric properties of ZST ceramics [23].
The excessive addition of La2O3 also diminished the dielectric properties of ZST ceramics. The trivalent La3+ behaved as an acceptor and may create the oxygen vacancies within the structure of a material according to the following expression:
The entrance of excess oxygen into the crystal structure of ZST ceramics impaired the homogeneity, leading to non-harmonic oscillations and decreased dielectric properties [33].
A comparison between the properties of undoped and doped ZST ceramics along with their sintering conditions, apparent densities, and microwave dielectric properties was provided in Table 3. The additives in the table varied in kind of the II A alkaline earth metal oxide (MgO, SrO, CaO and BaO). We found that adding a certain amount of MgO in our study not only conduced to reduce the sintering temperature of the ZST ceramics, but also demonstrated an insignificant decrease of Q value. Additionally, the temperature coefficients of the resonant frequency were − 1.23, 0.81, − 2.34, and 2.06 ppm/°C for LM1, LM2, LM3, and LM4 sintered at 1320 °C, respectively. The τf values had correlation to the ingredient and the impurity phases of the material [34]. ZST ceramics were temperature stable and dopants did not cause any impurity phases, the τf values did little alter with La2O3 and MgO contents added. According to the data listed in Table 3, LM2 sample seemed to be the most suitable for application in microwave components, due to the relatively lower sintering temperature (1320 °C), small temperature coefficient of the resonant frequency (τf equal to 0.81 ppm/°C), moderate relative permittivity (εr = 38.44), and excellent dielectric properties (Q × f value equal to 52,670 GHz at 5.6 GHz).
4 Conclusions
The structures and microwave dielectric properties of ZST ceramics with various contents of La2O3 and MgO were investigated. The XRD patterns indicated a homogeneous material composed of single phase with orthorhombic structure. The size of unit cell increased with the increased La2O3 and MgO contents, for all materials examined. The optimal contents of La2O3 and MgO dopants were 0.5 wt% and 1.0 wt%, respectively. This combination led to the sintered material with the relatively lower sintering temperature (1320 °C), improved density and microwave dielectric properties. The correlation between the MgO content and the morphology, density and dielectric properties of ZST ceramics was also confirmed in this study. The near zero τf values of ZST ceramics can be obtained and had no significant change under different contents of dopants. After being sintered at 1320 °C for 5 h, the excellent microwave properties: Q × f value of ~ 52,670 GHz (at 5.6 GHz), the εr value of ~ 38.44, and the τf value of ~ 0.81 ppm/°C, were obtained for ZST ceramics with 0.5 wt% La2O3 and 1.0 wt% MgO. The doped ZST material optimized in this study might be applied as a constituent of microwave components.
References
M. Xiao, Y.S. Wei, Q.Q. Gu, P. Zhang, The relationship between the bond ionicity, lattice energy, bond energy and microwave dielectric properties of LaNbO4 ceramics. J. Mater. Sci. Mater. Electron. 29(12), 9963–9970 (2018)
Q.L. Sun, H.Q. Zhou, X.F. Luo, L.S. Hu, L.C. Ren, Influence of La2O3/SrO doping of (Zr0.8Sn0.2)TiO4 ceramics on their sintering behavior and microwave dielectric properties. Ceram. Int. 42(10), 12306–12311 (2016)
D.D. Liu, P. Liu, B.C. Guo, Low-temperature sintering and microwave dielectric properties of Li4Mg3Ti2O9 ceramics by a sol–gel method. J. Mater. Sci. 29(12), 10264–10268 (2018)
S. Takahashi, A. Kan, H. Ogawa, Microwave dielectric properties and crystal structures of spinel-structured MgAl2O4 ceramics synthesized by a molten-salt method. J. Eur. Ceram.Soc. 37(3), 1001–1006 (2017)
R.Y. Yang, M.H. Weng, H. Kuan, TEM observation of liquid phase sintering in V2O5 modified Zr0.8Sn0.2TiO4 microwave ceramics. Ceram. Int. 35(1), 39–43 (2009)
N. Michiura, T. Tatekawa, Y. Higuchi, H. Tamara, Role of donor and acceptor ions in the dielectric loss tangent of (Zr0.8Sn0.2)TiO4 dielectric resonator material. J. Am. Ceram. Soc. 78(3), 793–796 (1995)
Y.C. Heiao, L. Wu, C.C. Wei, Microwave dielectric properties of (Zr,Sn)TiO4 ceramic. Mater. Res. Bull. 23(12), 1687–1692 (1988)
K. Wakino, K. Mino, H. Tamura, Microwave characteristics of (Zr,Sn)TiO4 and BaO-PbO-Nd2O3-TiO2 dielectric resonators. J. Am. Ceram. Soc. 67(4), 278–281 (1984)
Z.Y. Zhang, H.K. Zhu, Y. Zhang, Y.H. Chen, Z.X. Fu, K. Huang, Q.T. Zhang, Effects of the Ba3(VO4)2 additions on microwave dielectric properties of (Zr0.8Sn0.2)TiO4 ceramics. J. Mater. Sci. 28(2), 2044–2048 (2017)
Q.L. Sun, H.Q. Zhou, L. Qian, Y.Z. Wang, H.K. Zhu, Z.X. Yue, Effects of MgO, SrO and La2O3 co-doping on structure and properties of (Zr0.8Sn0.2)TiO4 ceramics. J. Inorg. Mater. 31(8), 812–818 (2016)
L. Qian, H.Q. Zhou, Q.X. Jiang, L.C. Ren, W.T. Xie, X.F. Luo, Q.L. Sun, Effect of MgO, BaO and La2O3 additions on microwave dielectric properties of (Zr0.8Sn0.2)TiO4 ceramics. J. Mater. Sci. 27(6), 6183–6187 (2016)
D.J. Kim, J.W. Hahn, G.P. Han, S.S. Lee, T.G. Choy, Effects of alkaline-earth-metal addition on the sinterability and microwave characteristics of (Zr,Sn)TiO4 dielectrics. J. Am. Ceram. Soc. 83(4), 1010–1012 (2000)
Z. Wang, Q.F. Shu, K.C. Chou, Structure of CaO-B2O3-SiO2-TiO2 glasses: a Raman Spectral Study. ISIJ. Int. 51(7), 1021–1027 (2011)
B. Chen, L. Han, B.Y. Li, X.D. Sun, Effects of CaO and La2O3 doping of (Zr0.8Sn0.2)TiO4 ceramics on the densifying behavior and microwave dielectric properties. J. Mater. Sci. 28(13), 9542–9547 (2017)
A. Ioachim, M.G. Banciu, M.I. Toacsen, L. Nedelcu, D. Ghetu, H.V. Alexandru, C. Berbecaru, A. Dutu, G. Stoica, High-k Mg-doped ZST for microwave applications. Appl. Surf. Sci. 253(1), 335–338 (2006)
B.W. Hakki, P.D. Coleman, A dielectric resonator method of measuring inductive capacities in the millimeter range. IEEE Trans. Microwave Theory Tech. 8, 402–410 (1960)
W.E. Courtney, Analysis and evaluation of a method of measuring the complex permittivity of microwave insulators. IEEE Trans. Microwave Theory Tech. 18, 476–485 (1970)
Y. Kobayashiy, M. Katoh, Microwave measurement of dielectric properties of low-loss materials by the dielectric rod resonator method. IEEE Trans. Microwave Theory Tech. 33, 586–592 (1985)
G.Q. Wang, S.H. Wu, H.Y. Yan, Study on the calcination and sintering technique of (Zr0.8Sn0.2)TiO4 ceramics. Piezoelectrics Acoustooptics. 25(4), 321–324 (2003)
L.Z. Wang, L.X. Wang, Z.F. Wang, B.Y. Huang, Q.T. Zhang, Z.X. Fu, Effect of ZnO/Er2O3 addition on microwave properties of (Zr0.8Sn0.2)TiO4 ceramics. J. Mater. Sci. 27(4), 3929–3933 (2016)
J.Q. Chen, Y. Tang, H.C. Xiang, L. Fang, H. Porwald, C.C. Li, Microwave dielectric properties and infrared reflectivity spectra analysis of two novel low-firing AgCa2B2V3O12 (B = Mg, Zn) ceramics with garnet structure. J. Eur. Ceram. 38(14), 4670–4676 (2018)
M. Mikoczyova, Influence of forming method and sintering process on densification and final microstructure of submicrometre alumina ceramics. Process. Appl. Ceram. 2(1), 13–17 (2008)
D. Pamu, G.L.N. Rao, K.C.J. Raju, Effect of BaO, SrO and MgO addition on microwave dielectric properties of (Zr0.8,Sn0.2)TiO4 ceramics. J. Alloy. Compd. 475(1–2), 745–751 (2009)
C.L. Huang, S.H. Huang, Low-loss microwave dielectric ceramics in the (Co1 – xZnx)TiO3 (x = 0-0.1) system. J. Alloy. Compd. 515, 8–11 (2012)
Q.S. Cao, W.Z. Lu, X.C. Wang, J.H. Zhu, B. Ulla, W. Lei, Novel zinc manganese oxide-based microwave dielectric ceramics for LTCC applications. Ceram. Int. 41(9), 9152–9156 (2015)
Z.Y. Zou, Z.H. Chen, X.K. Lan, W.Z. Lu, B. Ulla, X.H. Wang, W. Lei, Weak ferroelectricity and low permittivity microwave dielectric properties of Ba2Zn(1 + x)Si2O(7 + x) ceramics. J. Eur. Ceram. Soc. 37(9), 3065–3071 (2017)
X.S. Lyu, L.X. Li, H. Sun, S. Zhang, S. Li, High-Q microwave dielectrics in wolframite magnesium zirconium tantalate ceramics. Ceram. Int. 42(1), 2036–2040 (2016)
R.D. Shannon, Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phys. 73(1), 348–366 (1993)
L. Fang, C.X. Su, H.F. Zhou, Z.H. Wei, H. Zhang, Novel low-firing microwave dielectric ceramic LiCa3MgV3O12 with low dielectric loss. J. Am. Ceram. Soc. 96(3), 688–690 (2013)
S.X. Zhang, J.B. Li, H.Z. Zhai, J.H. Dai, Low temperature sintering and dielectric properties of (Zr0.8Sn0.2)TiO4 microwave ceramics using La2O3/BaO additives. Mater. Chem. Phys. 77(2), 470–475 (2002)
W.S. Kim, T.H. Kim, E.S. Kim, K.H. Yoon, Microwave dielectric properties and far infrared reflectivity spectra of the (Zr0.8Sn0.2)TiO4 ceramics with additives. Jpn. J. Appl. Phys. 37(9B), 5367–5371 (1998)
Q.L. Sun, H.Q. Zhou, H.K. Zhu, H.Q. Qi, L.S. Hu, Z.X. Yue, Sintering behavior and microwave dielectric properties of Y2O3-ZnO doped (Zr0.8Sn0.2)TiO4 ceramics. J. Mater. Sci. 27(8), 7750–7754 (2016)
R.K. Bhuyan, T.S. Kumar, D. Goswami, A.R. James, D. Pamu, Liquid phase effect of La2O3 and V2O5 on microwave dielectric properties of Mg2TiO4 ceramics. J. Electroceram. 31(1–2), 48–54 (2013)
L.Z. Wang, L.X. Wang, Z.F. Wang, B.Y. Huang, Q.T. Zhang, Z.X. Fu, Effect of sintering aid ZnO-CeO2 on dielectric properties of (Zr0.8Sn0.2)TiO4 ceramics. J. Mater. Sci. 26(11), 9026–9030 (2015)
Acknowledgements
This work was supported by National Natural Science Foundation of China (No.51578327).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, B., Han, L. & Li, B. Sintering characteristics and microwave dielectric properties of (Zr0.8Sn0.2)TiO4 ceramics doped with La2O3 and MgO. J Mater Sci: Mater Electron 30, 2847–2853 (2019). https://doi.org/10.1007/s10854-018-0561-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0561-4