Abstract
Cerium modified bismuth titanate ceramics were studied as potential lead-free ferroelectric materials over a broad temperature range. Polycrystalline samples of Bi4−xCexTi3O12 (x = 0.2, 0.4, 0.6, 0.8) (BCeT) were prepared using the solution combustion technique. The effect of Ce doping on their crystalline structure, ferroelectric properties and electrical conduction characteristics were explored. An increase in the dielectric constant and decrease in loss tangent is observed due to addition of Ce in bismuth titanate lattice up to a certain threshold doping concentration. The presence of pyrochlore phase decreases the density of ceramics with composition x = 0.8 which may be due to grain inhibitor property of Ce. The value of remnant polarization increases with increasing doping content up to x = 0.6 due to decrease in defect sites in the samples, further increase in cerium content induces leakage current which makes the sample lossy. The real–imaginary impedance plots are interpreted using RC model, this explains the contribution of the grain-bulk and the grain boundary resistivities to the total resistivity of the materials. Activation energy increases with increasing Ce concentration indicating a decrease in defect sites due to which the conductivity of the doped ceramics reduces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bismuth layer structured ferroelectrics (BLSF) belonging to Aurivillius family have attracted technologists owing to their applications in electronic devices like ferroelectric random access memory (FeRAM), actuators, transducers and micro-electro-mechanical systems (MEMS) [1, 2]. Due to environmental concerns, lead free materials are being considered for such applications and Bismuth Titanate (Bi4Ti3O12) (BIT) is one of the few materials that are in forefront of non-lead-based ferroelectric materials [3]. Crystalline structure of Bi4Ti3O12 comprises of (Bi2Ti3O10)2− layers formed by BiTiO3 unit cells of perovskite like structures alternating with (Bi2O2)2+ layers perpendicular to c-axis [4]. It has high Curie temperature (Tc) and good fatigue endurance. However, its electrical conductivity is high due to which the poling process becomes tedious [5]. This predicament can be overcome by adopting a low temperature synthesis route and by densification of randomly oriented grains which is necessary to control the conductivity conduit along the layers [6].
Previous studies [7, 8] reveal that dielectric, electrical and ferroelectric properties can be competently tailored by using various processing route and optimizing synthesis parameters by which microstructure of the material can be improved. This has significant influence on the dielectric and ferroelectric properties of the material. In addition to this, modification of the basic compound by ion substitution either at A-site or B-site in BIT can also enhance its properties. Thus, the influence of A site substitution with isovalent La3+, Nd3+, Pr3+ and Sm3+ on dielectric and ferroelectric properties has been well studied [9]. It is reported [10] that the polarization of the system can be enhanced by doping with element of appropriate ionic radii of lanthanide series.
Palovic et al. [11] reported the structure and morphology of Ce doped bismuth titanate ceramics obtained by co-precipitation route and studied its effect on dielectric properties [12]. However, the influence of cerium addition in bismuth titanate lattice on its dielectric, electrical and ferroelectric properties still needs to be explored and is yet to be reported in the literature.
Impedance spectroscopy is a powerful tool to analyze grain and grain boundary contribution to electrical conductivity in ferroelectric materials [13]. By using an equivalent circuit of resistance and capacitance for grain and grain boundary separately, the electrical properties of the material can be determined. This study also gives information regarding the role of defects in the A.C. response under given conditions and also concerning the relaxation mechanism [14].
Thus, a methodical impedance study is taken up in the present investigation to understand the effect of Ce3+ cations on A-site of the samples. The understanding of ionic relaxation is supposed to give an insight about the frequency-temperature-impedance data for the development of electronic devices. In this paper we report the effect of cerium modification of Bismuth Titanate ceramics with general formula Bi4−xCexTi3O12 (x = 0.2, 0.4, 0.6, 0.8) on phase formation, morphology, dielectric, ferroelectric properties and impedance spectroscopic analysis.
2 Experimental
2.1 Synthesis
Polycrystalline samples of Ce (A site) doped Bismuth Titanate (Bi4Ti3O12) (BCeT) with different doping concentrations of Ce were synthesized using the solution combustion technique. Bi(NO3)3.5H2O (Merck), Ce(NO3)3.6H2O (Sigma Aldrich) and C12H28O4Ti (Sigma Aldrich) were used as starting materials in stoichiometric ratios. All the raw materials used were AR grade having 99.9 % purity. The procedure adopted for preparation of these ceramics is same as that reported in our earlier publication [15].
2.2 Characterization
The ceramics were examined for the existence of different phases using X-ray diffractometer (Rigaku—Miniflex II) equipped with Cu Kα (λ = 1.54 Å) radiation. The experimental density of the as prepared ceramics was determined using Archimedes Principle (ASTM# C 373-88). Scanning Electron Microscope (JEOL—JSM6390) was used to study the surface morphology of the pellets. Both the surfaces of the pellets (circular discs of diameter 10 mm (approx) and thickness 1 mm (approx)) were coated with a layer of Platinum using sputter coating process for the formation of ohmic contacts. The dielectric measurements were carried out using Alpha High Resolution Dielectric impedance analyzer using Nova Controls in the frequency range from 1 Hz to 1 MHz from room temperature to 750 °C. PE Loop measurements were performed using PE Loop tracer (Radiant Technology) at a frequency of 50 Hz and a maximum field of 20 kV/cm at room temperature.
3 Results and discussion
3.1 X-ray diffraction studies
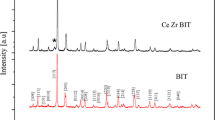
Information pertaining to the structure, phases and effect of Ce doping on the crystallinity of BIT powders, was obtained from the X-ray diffraction patterns shown in Fig. 1a. It was observed that X-ray diffraction patterns of bismuth titanate powders with different contents of cerium (x = 0, 0.2, 0.4, 0.6, and 0.8) confirm the existence of two different phases (i) Aurivillius Bismuth titanate layered structured phase and (ii) cubic pyrochlore (marked with *) phase (Bi2Ti2O7) as per JCPDS file no. 32-0118. It is noted that as the doping concentration increases the intensity of the peaks corresponding to the pyrochlore phase increases. The presence of pyrochlore phase with rising cerium content indicates that the cerium has been incorporated into the bismuth titanate structure, which was also reported earlier by Palovic et al. [11]. Cerium ions can be incorporated into bismuth titanate lattice in two oxidation states as they can exist as Ce3+ and Ce4+. In oxidation state 4+ it could substitute titanium at Ti4+ sites whereas in oxidation state 3+ it could be incorporated at Bi3+ site [16]. Considering the ionic radii of Ce3+ (1.01 Å) and Ce4+ (0.87 Å) it can be inferred that the probability of Ce3+ occupying Bi3+ (1.03 Å) site is more as the Hume Rothery criterion for solid solutions says the difference in the ionic radii of host cation and substituting cation should not be greater than 15 %. The XRD patterns of Bi4Ti3O12 crystals were indexed based on those given in JCPDS data cards file no. 72-1019 and was found to match well with the said JCPDS file which confirms the formation of BCeT. It was observed that, the as-prepared samples have orthorhombic symmetry with end-centered lattice. A magnified look at the major peak (117) shown in Fig. 1b indicates shift in the peak position corresponding to the plane [117] towards higher values of 2θ signifying change in the volume of the unit cell of bismuth titanate due to substitution of Bi3+ by Ce3+.
3.2 Density measurements
Density of the prepared electroceramics has a pronounced effect on its properties. It is noted that the Ce doped bismuth titanate samples sintered at 1050 °C for 5 h showed experimental density in the range 7.28–7.51 gm/cm3 (exact values are listed in Table 1) as compared to theoretical density (ρth = 8.04 gm/cm3). The lower values of density can be attributed to the porosity introduced in the powders during the combustion process. However, the formation of fairly uniform grain structure is discussed in the next section.
3.3 SEM analysis
SEM micrographs of bismuth titanate ceramics prepared with different amount of cerium doping, sintered at 1050 °C are shown in Fig. 2. The morphology consists of plate like grains which are mostly anisotropic. It is observed that as the value of x in the composition Bi4−xCexTi3O12 increases, the grain size of these samples decreases. Length of grains decreases from 30 μm for x = 0.2 to around 5 μm for x = 0.8 while the thickness varies from 5 to 1 μm. It is also noted that the grain size distribution is more uniform in the sample with higher cerium content which may be due to the fact that the fine grains that exist between large anisotropic grains become larger with higher x value; these observations are in agreement with that of Tang et al. [17]. The fine grains existing between the large anisotropic grains may be of pyrochlore phase. This suggests that addition of small amount of cerium controls the grain growth in BIT ceramics. Change in the microstructure with doping concentration can be attributed to the inhibition of the Bi3+ volatilization by substitution of Ce3+ and believed to be due to the reported formation of cubic pyrochlore phase bismuth titanate as observed in XRD results reported in Sect. 3.1. Pyrochlore phase inhibits grain growth till Ce diffuses into BIT lattice up to a certain Ce concentration i.e. x = 0.6. On further increasing the Ce content (for x = 0.8) the grains formed in pyrochlore phase increases which decreases the density of the ceramic.
3.4 Dielectric properties
The temperature dependence of dielectric constant of Ce doped Bismuth titanate ceramics at different frequencies are shown in Fig. 3. It can be observed that the ferroelectric phase transition temperature Tc shifts towards lower temperature side with the increase in doping concentration. This may be a consequence of incorporation of Ce in Bi4Ti3O12 lattice, which causes crystal lattice distortion [18]. The value of dielectric constant at Curie temperature increases with the increase in doping level. The possible reasons for this could be the creation of structural defects due to the unique nature of Ce of changing its oxidation state from +3 to +4 and the presence of secondary Bi2Ti2O7 phase. As the doping concentration is increased, the εr versus temperature curves show diffuse phase transition, which is the characteristic of a relaxor ferroelectric behavior. In pure BIT, an anomalous peak is observed [15] around 500 °C in addition to the peak around Tc, prominently at lower frequencies, whereas there is no such peak in any of the doped specimens. As the reason for this anomaly is attributed to the space charges generated by bulk or by surface of the crystal [19, 20] due to the presence of defects or oxygen vacancies. It can be concluded that addition of small amount of Ce reduces such defects or vacancies. Table 1 summarizes the porosity corrected dielectric constant of Ce modified bismuth titanate ceramics with different doping concentrations. Porosity correction for the measured dielectric constant was carried out using Rushman and Strivens equation [21]:
where V2 is the volume fraction of porosity in the sintered compact.
Room temperature porosity corrected dielectric constant values for all the samples are listed in Table 1. It can be seen that dielectric constant increases with the doping concentration and reaches maximum at x = 0.6. This rise in the value of dielectric constant may be due to increase in density of the ceramics and reduction in the vacancies created due to Bi3+ volatilization as Ce3+ occupies Bi3+ sites and the pyrochlore phase present in these samples is very less hence it does not affect its dielectric properties. On further increase in the doping level the value of ε is found to decrease. This decrease in dielectric constant may be attributed to the significant rise in the pyrochlore phase present within the sample which reduces the density and also deteriorates the dielectric properties. The presence of pyrochlore phase was also confirmed with XRD analysis in Sect. 3.1.
The dielectric loss of BCeT ceramics as a function of temperature at different frequencies are shown in Fig. 3, which demonstrates that the values of tanδ are very low at lower temperatures for all frequencies and it increases at elevated temperatures for all doping concentrations. No significant influence of Ce doping on dielectric losses was observed up to the transition temperature Tc. Sharp rise in the value of dielectric loss above Tc was observed and is due to space charge polarization. Above Curie temperature the conductivity begins to dominate thereby increasing the value of tanδ [22]. The value of tanδ of BCeT samples were much lesser than that of pure BIT, this may be attributed to decrease in space charge density as Ce incorporates in BIT lattice.
3.5 Impedance analysis
The Nyquist plots for the compounds Bi4−xCexTi3O12 (x = 0, 0.2, 0.4, 0.6, 0.8) are represented by Fig. 4. Complex impedance behavior of these ceramics can be explained by a series of three parallel RC circuit elements that match with the dielectric behavior of the grain-bulk, the grain-boundary, and the electrode-surface respectively. Each RC element usually corresponds to a semicircle. These semicircles can be resolved into two or three each, depending on the frequency region. The grain boundary effect is represented by the lower frequency semicircles and the high frequency ones correspond to the grains [23]. Each of the semicircle or each RC element with their associated parameters, grain resistance (Rg), grain capacitance (Cg), grain boundary resistance (Rgb), grain boundary capacitance (Cgb), and electrode surface resistance (Re), electrode surface capacitance (Ce), represents the resistance and the capacitance of the respective regions. The frequency corresponding to the maximum of each semicircle corresponds to the relaxation frequency and the radius of the semicircle gives the resistance. The centers of the semicircles are positioned below the real impedance axis (Z′), which is indicative of the existence of two or more relaxing charge defects and consequently suggesting that the as prepared samples do not follow Debye’s relaxation behavior but follows Maxwell–Wagner (M–W) relaxation behavior [24].The decrease in the radius of the semicircles with the rise in temperature, indicates the decrease in relaxation time.
The experimental impedance data is fitted to an equivalent circuit using Electrochemical Impedance Spectroscopy (EIS) software. For comprehensive study of the spectra a simplified equivalent circuit of resistors in series, each shunted by constant phase element (CPE), is used as shown in Fig. 4. CPE represents the inhomogeneous distribution of grains and their sizes. It is observed that for Ce doped samples (Fig. 4a–d) only two semicircles were obtained indicating the electrode surface resistance Re to be negligible as compared to Rg and Rgb. This implies that addition of small amount of Ce in pure BIT increases the resistivity due to grains and grain boundaries in a manner that the resistance due to electrode surface interface becomes negligible in comparison. The grain resistance (Rg) and grain boundary resistance (Rgb) can be obtained from the intercept on real (Z′) impedance axis. Also, the capacitances (Cg and Cgb) can be obtained from the frequency corresponding to the highest point in each semicircle, using the relation 2fmaxRC = 1; where fmax is the relaxation frequency. It is observed that Rg ≪ Rgb which indicates that in the overall electrical behavior of the as prepared ceramics, the grain boundary resistance plays a dominant role.
The variation of imaginary part of impedance (Z″) with frequency at different temperatures for different doping concentrations is shown in Fig. 5. It is observed that initially the value of Z″ increases up to a maximum value (Zmax) and then it decreases with further rise in frequency for all temperatures and for all doping concentrations. It is also noticed that Zmax value reduces with rise in temperature, denoting the increase in capacitance and decrease in resistance (i.e., rise in conductivity) of the material. Also, as the temperature is raised, the peak tends to broaden for all the samples as shown in Fig. 5 and the frequency corresponding to Zmax shifts towards higher frequency side. The broadening of peak signifies multiple relaxations in the material. The cause of multiple relaxations is the presence of electrons or immobile species at lower temperatures and structural changes at higher temperatures [20]. Frequency corresponding to Zmax is different for different concentrations of Ce indicating difference in the relaxation times for each of the sample. The curves merge into one at higher frequencies for all doped concentrations indicating the decrease in space charge effects at higher frequencies [25] whereas in undoped BIT [15] the curves do not merge at all even at higher frequencies.
The plot of scaled Z″ versus reduced frequency (f/fmax) for all the doping concentrations is depicted in Fig. 6. It can be observed that the scaled data points combine into a master curve for all the samples with different doping content. The value of full-width at half-maximum (FWHM) is found to be >1.14 decades. From these observations it can be concluded that there exists time independent relaxation distribution function with non-exponential conductivity relaxation, due to which there is a probability of ion movement and time dependent mobility of other charge carriers of the similar kind in the neighborhood [26]. In the lower frequency range, the charge carriers can jump for one site to another neighboring site over long distances, and in the higher frequency range the localized movements of the charge carriers are restricted within the potential wells. The frequency region in which the peak is observed is the transition region from long range to short range movement of charge carriers. The perfect overlapping of all the curves at different temperatures for all the frequencies implies that all relaxation mechanisms taking place possess same thermal energy and exhibit temperature dependent dynamical processes [27].
Variations of relaxation time with inverse of temperature are presented in Fig. 7. The relaxation frequency can be obtained from: (i) plot of Z″ versus log of frequency and (ii) semicircles obtained by Nyquist plots. In the current work the relaxation time is calculated from the peak position of Z″ vs. frequency plot (Fig. 5) using the relation 2πf maxτ = 1, where f max is the relaxation frequency and is independent of the geometrical parameters of the sample. The plot τ versus 1/T gives a straight line and can be approximated to the relation [28]
where τ0 is the pre-exponential factor, Ea is the activation energy and kB is the Boltzman constant. The activation energy for relaxation as evaluated from the graphs for Bi4−xCexTi3O12 (x = 0.2, 0.4, 0.6, 0.8) and are given in Table 1.
3.6 Electrical properties
The change in electrical conductivity with frequency at different temperatures is presented in Fig. 8. It is clear from these figures that the conductivity increases with temperature. At lower frequencies a plateau is observed where the conductivity remains constant with frequency and at higher frequencies the conductivity varies linearly with frequency which is called as the dispersion region. The change in the behavior of conductivity from being ‘frequency independent’ to ‘frequency dependent’ is observed at a particular frequency called hopping frequency and is observed that it shifts towards higher frequency side with the rise in temperature. In the dispersion region, the rise in conductivity is due to hopping of charge carriers in finite bunches. In the plateau region, the conductivity may be due to the successive jumping of mobile ions from one place to other adjacent site. This is also termed as long-range transport of mobile ions in response to the applied field [29]. The conductivity as a function of frequency obeys Jonscher’s power law [30] given by
s is the frequency exponent which ranges between 0 and 1. In this case, A and s are temperature dependent parameters and indicate that electrical conduction is a thermally activated process and ω is the angular frequency (2πf) of the applied AC field. Value of s has physical significance in the sense that for higher values of s (closer to 1), the hopping motion goes along with either translational or rotational motion of the mobile species [31]. The frequency independent part of the curve in the lower frequency region represents the dc conductivity (σdc) and the frequency dependent part in the higher frequency region can be explained by the term Aωs. Values of s are calculated from the slope of σ − f plots in the higher frequency region. Figure 9 shows the variation of s with temperature for all doping concentrations of BIT. It is observed that s decreases with the increase in temperature, which is in accordance with the correlated barrier hopping (CBH) theoretical model [32]. The temperature dependence of s indicates that the conduction mechanism in these materials is not due to the intrinsic polarization process, but is a thermally activated phenomenon which is associated with the hopping of charged species across the charged defects. A charge carrier trapped in a lattice within the distortion produced by its presence in the localized site is known as polaron [24].
Variations of conductivity with inverse of temperature (Arrhenius plots) for Ce doped ceramics of all doping concentrations are shown in Fig. 10. It is observed that the conductivity increases with increase in temperature due to rise in oxygen vacancies with temperature. Also it is noted that at higher temperatures (200–600 °C) the conductivity varies linearly with temperature and at lower temperatures (RT-100 °C) it becomes almost constant. This type of behavior is in accordance with Motts hopping phenomenon [33]. The activation energy for all the Ce doped samples is calculated using Arrhenius equation
and the values are given in Table 1. It is noted that activation energy increases with increase in doping concentration indicating decrease in conductivity of the ceramic with increase in doping level because Ce substitution in BIT decreases the defects due to oxygen vacancies or Bi3+ ion volatilization. Also, the values of activation energies due to relaxation are found to be greater than activation energies due to conduction, indicating that the conduction is not governed by relaxation mechanism (Table 2).
3.7 Ferroelectric properties
The ferroelectric properties of sintered Bi4−xCexTi3O12 (x = 0, 0.2, 0.4, 0.6, 0.8) ceramics measured at 50 Hz at room temperature under the maximum applied field of 10 kV/cm are shown in Fig. 11. Remnant polarization (2Pr) and coercive field (2Ec) both were found to increase with the increase in the doping concentration It is observed that remanent polarization (2Pr) increases with increase in doping content, this can be attributed to decrease in defect sites in the sample due to addition of cerium which suppresses the Bi3+ volatilization and also reduces the oxygen vacancies in the as prepared samples [34]. The hysteresis loop corresponding to x = 0.8 is not saturated and is lossy in nature. The probable reason behind this degradation is low density and higher leakage current. As seen from Table 1 there is a difference of two orders in the value of resistivity of the sample corresponding to x = 0.8. This suggests that at x = 0.8 more space charge will contribute in the lattice and increases the conductivity as a result a lossy P–E loop is observed.
4 Conclusions
Therefore, it is concluded that Ce modified bismuth titanate samples can be successfully synthesized by solution combustion route. An XRD study confirms the presence of pyrochlore phase along with pure BIT phase indicating the incorporation of Ce in the BIT structure. The grain size of the Ce doped samples decreases with increase in amount of cerium addition due to formation of additional pyrochlore phase. Also the ferroelectric transition temperature (Tc) decreases and dielectric constant increases with increasing doping concentration. The compounds show temperature dependent relaxation process. The activation energy for relaxation and conduction was calculated. The impedance plots suggest that the as prepared samples follow Maxwell–Wagner (M–W) relaxation behavior. The behavior of conductivity with temperature suggests that the conduction mechanism is thermally activated at higher frequencies. The remnant polarization tends to increase with increased doping content due to decrease in defect sites in the samples, but addition of Ce beyond a certain threshold level induces leakage current due to decrease in the density of the ceramic which makes the sample lossy. Hence addition of small amount of cerium in bismuth titanate enhances its dielectric and ferroelectric properties.
References
D. Guo, M. Li, J. Wang, J. Liu, B. Yu, B. Yang, Appl. Phys. Lett. 91, 232905 (2007)
A. Wu, M. Roasa Soares, I.M. Miranda Salvado, P.M. Vilarinaho, Mater. Res. Bull. 47, 3819–3824 (2012)
Z.S. Macedo, C.R. Ferrari, A.C. Hernandes, Powder Technol. 139, 175–179 (2004)
B. Aurivillius, Ark. Kemi. 1, 499 (1949)
N. Azurmendi, I. Caro, J. Am. Ceram. Soc. 89(4), 1232–1236 (2006)
M. Anil Kumar, S.R. Dhage, V. Ravi, Mater. Lett. 59, 514–516 (2005)
L.B. Kong, J. Ma, W. Zhu, O.K. Tan, Mater. Lett. 51(2), 108–114 (2001)
B.D. Stojanovic, A.Z. Simoes, C.O. Paiva-Santos, C. Quinelato, E. Longo, J.A. Varela, Ceram. Int. 32(6), 707–712 (2006)
M. Villegas, T. Jardil, A.C. Caballero, J.F. Fernandez, J. Electroceram. 13, 543–548 (2004)
K.-T. Kim, C.-I. Kim, Mater. Sci Eng. B 118, 229–233 (2005)
N. Palovic, V.V. Srdic, Mater. Res. Bull. 44, 860–864 (2009)
N. Pavlovic, V. Koval, J. Dusza, V.V. Srdic, Ceram. Int. 37, 487–492 (2011)
M.E. Lines, A.M. Glass (Claredon Press, Oxford, 1977)
N.V. Prasad, S.N. Babu, A. Siddeshwar, G. Prasad, G.S. Kumar, Ceram. Int. 35, 1057–1062 (2009)
O. Subohi, L. Shastri, G.S. Kumar, M.M. Malik, R. Kurchania, Mater. Res. Bull. 49, 651–655 (2014)
S.K. Singh, H. Ishiwara, J. Sol-Gel. Sci. Technol. 42, 231–238 (2007)
Q.Y. Tang, Y.M. Kan, Y.G. Li, G.J. Zhang, P.L. Wang, Scr. Mater. 54, 2075–2080 (2006)
K. Babooram, D.K. Chin, Z.G. Ye, J. Electroceram. 21, 43–48 (2008)
J.D. Bobic, M.M. Vijatovic Petrovic, J. Banys, B.D. Stojanovic, Mater. Res. Bull. 47, 1874–1880 (2012)
G.S. Lotey, N.K. Verma, J. Nanopart. Res. 14(3), 1–11 (2012)
D.F. Rushman, M.A. Strivens, Proc. Phys. Soc. 59, 1011 (1947)
B. Mamtha, M.B. Suresh, A.R. James, M. Vithal, P. Sarah, Phys. Scr. 84, 055704 (2011)
C.S. Devi, M.B. Suresh, G.S. Kumar, G. Prasad, J. Adv. Dielectr. 2(1), 1250001–1250014 (2012)
O. Subohi, G.S. Kumar, M.M. Malik, R. Kurchania, Phys. B 407(18), 3813–3817 (2012)
T. Badapanda, V. Senthil, S.K. Rout, S. Panigarhi, T.P. Sinha, Mater. Chem. Phys. 133, 863–870 (2012)
K. Prasad, A. Kumar, S.N. Choudhary, R.N.P. Choudhary, Solid State Ionics 176, 1641–1646 (2005)
S. Saha, T.P. Sinha, Phys. Rev. B 65, 134103 (2002)
L.K. Kumari, K. Prasad, R.N.P. Choudhary, J. Alloys Compd. 453, 325–331 (2008)
S. Dutta, S. Bhattacharya, D.C. Agarwal, Mater. Sci. Eng. B 100(2), 191–198 (2003)
M. Vijaykumar, S. Selvasekarapandian, M.S. Bhuvaneswari, G. Hirankumar, G. Ramprasad, R. Subramanian, P.C. Angelo, Phys. B 334, 390–397 (2003)
S. Sen, R.N.P. Choudhary, Mater. Chem. Phys. 87, 256–263 (2004)
Shrabanee Sen, P. Pramanik, R.N.P. Choudhary, Ceram. Int. 33(4), 579–587 (2007)
S. Mollah, K.K. Som, K. Bose, B.K. Chaudri, J. Appl. Phys. 74, 931 (1993)
G.S. Lotey, N.K. Verma, Mater. Lett. 111, 55–58 (2013)
Acknowledgments
Authors are thankful to the Director, MANIT Bhopal, for providing the infrastructure to carry out this research work and also thank Dr. V. Raghavendra Reddy DAE-CSR, Indore, for providing P–E loop facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subohi, O., Singh, R., Kumar, G.S. et al. Impedance analysis and dielectric properties of Ce modified bismuth titanate lead free ceramics synthesized using solution combustion route. J Mater Sci: Mater Electron 26, 9122–9133 (2015). https://doi.org/10.1007/s10854-015-3600-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3600-4