Abstract
Ceramics of bismuth titanate, Bi4Ti3O12 (BIT) and the La-doped series, Bi4−x La x Ti3O12 (xBLT) with x = 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.75, have been synthesized by a new sol-gel process based on ethylene glycol. La-doping is found to reduce the temperature of the formation of pure Bi-layer-structured phase from 600 °C in BIT and low La-doped xBLT (x = 0.1–0.3) to 500 °C in high La-doped xBLT (x = 0.4–0.75). Increasing the La-content in the xBLT ceramics decreases the contribution of the space charge polarization to the apparent dielectric permittivity. The ceramics of xBLT prepared by this sol-gel route exhibit improved dielectric properties, with a higher room temperature dielectric constant and lower losses up to high temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quest for ferroelectric materials for application in non-volatile ferroelectric random access memory devices (FRAM) has been intensively pursued. Initially, the low processing temperature and high remanent polarization of Pb(Zr x Ti1−x )O3 [PZT] made it the leading material for memory devices [1], which is still in use in current FRAM. However, alternative materials are urgently needed because of some drawbacks of PZT, such as fatigue, leakage current, and aging [2, 3]. Another disadvantage of PZT is its high lead content which in the long term may cause major environmental and health concerns. As such, SrBi2Ta2O9 (SBT) was found to be a promising alternative with its excellent fatigue resistance and very good data retention over a long period of time [1, 4], but the relatively low remanent polarization and the higher processing temperature of SBT may limit its application in FRAM [5]. This has led to research on other lead-free ferroelectric materials with better properties.
Bi4Ti3O12 (BIT) has emerged as a possible solution to the problem. BIT belongs to the Aurivillius family of compounds [6] with a layered structure in which n perovskite-like (An−1B n O3n+1)2− blocks alternate with (Bi2O2)2+ layers. In BIT, three perovsite (Bi2Ti3O10)2− units are stacked with the bismuth–oxygen layers, and the major component of spontaneous polarization is along the a-axis (only a much smaller component along the c-axis). As a lead-free ferroelectric with a high Curie temperature (T C = 675 °C) and a high remnant polarization (P r = 50 μC/cm2) along the main polar crystallographic a-axis [7], BIT appears as a promising candidate for applications in piezoelectric transducers and memory devices in a wide temperature range [8, 9]. However, BIT, especially in the form of thin film, is known to suffer from serious fatigue failure after ∼106 switching cycles only [10]. In the wake of intensive research efforts, it was found that replacing some of the Bi3+ ions in the perovskite slabs by the La3+ ions greatly improves the properties of BIT and also lowers the processing temperature [11]. For instance, thin films of Bi4−x La x Ti3O12 (x = 0.75) were prepared at a relatively low temperature of 650 °C, which exhibit good fatigue endurance even after 3 × 1010 switching cycles [12]. With its lower processing temperature and higher remanent polarization compared to SBT, Bi3.25La0.75Ti3O12 stands as a better candidate for FRAM application.
The high T C in BIT results in a large coercive field (E c) at room temperature, making the poling of the material difficult. To lower E c, T C must be decreased. Wolfe and Newnham [13] found that substituting trivalent rare earth ions for Bi3+ in the perovskite slabs of the Bi-layered ferroelectrics has the effect of decreasing T C. Takenaka and Sakata [11] studied the effect of the La3+ substitution for Bi3+ in BIT, i.e. Bi4−x La x Ti3O12 (xBLT), and found that T C decreases with the increasing amounts of La3+ from 675 °C for x = 0 to about −150 °C for x = 1.5. The remanent polarization slightly decreases when x increases from 0 to 0.25. It then increases until a maximum value is attained at x = 0.75, after which it decreases considerably. The coupling constant also reaches a maximum value at x = 0.75.
A number of different techniques were reported for the synthesis of BIT and BLT ceramics and thin films. The metal-organic chemical vapor deposition, the metal-organic deposition, and the sol-gel process are among the most commonly used methods for preparing the thin films of these materials. Solid state reactions employed for the synthesis of BIT powders require relatively high temperatures (>1,100 °C) [14]. Several soft chemical routes were used for the preparation of BIT ceramics, including the co-precipitation method [15, 16], molten salt synthesis [17], and sol-gel process [18, 19]. In contrast, there have been only a few reports on the soft chemical synthesis of BLT powders and ceramics. Shen et al. [20] reported the sol-gel preparation of Bi4−x La x Ti3O12 (x = 0.4) powders and ceramics using ethylene glycol as a solvent. This method allowed the preparation of fine BLT powders at a calcination temperature of 750 °C. However, it required drying the sol at 95–105 °C over a period of 5 days in order to obtain the gel.
In this paper, we report the synthesis of Bi4Ti3O12 and Bi4−x La x Ti3O12 ceramics by an improved sol-gel method developed based on ethylene glycol following our recent work on the soft chemical synthesis of SBT ceramics [21]. Our main objective is to study the effect of sol-gel processing parameters and La-doping on the phase formation and dielectric properties of the BLT compounds. It is shown that, once again, ethylene glycol proves to be a good solvent for the starting materials, and this new method allows the synthesis of the xBLT powders and ceramics at relatively low temperatures with good dielectric properties.
Experimental
The ceramics of Bi4Ti3O12 [BIT] and Bi4−x La x Ti3O12 [xBLT] (with x = 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.75) were synthesized by a new sol-gel method using ethylene glycol as a solvent. Figure 1 outlines the flowchart of this process. Stoichiometric amounts of bismuth (III) acetate [Bi(OOCCH3)3] and titanium di-isopropoxide bis-acetyl acetonate (TIAA) [Ti(OC3H7)2(O2C5H9)2] were separately refluxed in ethylene glycol for about 1 h until a clear solution was obtained in each case. About 10 ml of acetic acid was also added to the bismuth solution during the reflux step in order to avoid precipitation. The two clear solutions were then mixed together and refluxed for about 1/2 h. The resulting orange Bi–Ti solution was then dried under vacuum on a rotary evaporator to produce a gel which was then treated at 500 °C for 2 h to produce a yellow BIT precursor powder. For the xBLT compounds, the above procedure was kept the same except that stoichiometric amounts of lanthanum (III) 2,4-pentanedionate [La(O2C5H9)3] were refluxed in ethylene glycol for about 1 h in a separate flask, and the resulting clear solution was added to the Bi–Ti mixture. After further refluxing for 1 h, clear yellow Bi–Ti–La sols were obtained which were then dried using the same procedure as used for the Bi–Ti sol to produce BLT gels and, finally, the BLT precursor powders.
The BIT and BLT precursor powders were ground with acetone, pressed into discs, and calcined at different temperatures to form pure phase compounds. After calcination, the pellets were ground and a few drops of a solution of polyvinyl alcohol were added as binder. The mixture was ground to a fine powder and pressed into pellets which were first heated up to 500 °C and kept for 1 h in order to burn away the binder, and then sintered at 900 °C for 8 h in a double crucible set-up containing a Bi2O3 atmosphere in order to minimize bismuth oxide loss.
The phases of the BIT and BLT samples were checked by X-ray powder diffraction using \({\text{CuK}}_{\alpha }\) radiation (46 kV, 42 mA) on a Rigaku X-ray diffractometer. The dielectric permittivity was measured as a function of temperature (30–500 °C) in a frequency range of 102–106 Hz on a computer-controlled Solartron impedance analyzer in conjunction with a dielectric interface.
Results and discussion
Phase analysis by X-ray diffraction
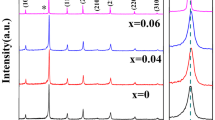
The X-ray diffraction (XRD) patterns for the BIT samples calcined at different temperatures are shown in Fig. 2(a). It can be seen that crystallization already occurs at 500 °C, with the presence of the Bi-layer-structured phase and small amounts of impurity phases, namely Bi2Ti2O7, Bi12TiO20, and TiO2. Upon increasing the temperature to 600 °C, the BIT phase grows while the impurity phases have all disappeared. The pure BIT phase remains stable up to 900 °C. At 1,000 °C, however, the Bi12TiO20 phase reappears in a small amount (together with an unknown peak at 2θ ≈ 46°). This observation is consistent with the report by Hardy et al. on the formation of BIT from an aqueous metal-chelate gel [22]: the pure phase of BIT occurred at 625 °C, however, heating above 700 °C led to the formation of secondary phases.
Figure 2(b) shows the XRD patterns of xBLT (with x = 0.1–0.75) synthesized at 500 °C. The xBLT samples with x = 0.1, 0.2, and 0.3 contain a mixture of the Bi-layered phase and some impurity phases, similar to the BIT sample synthesized at 500 °C, but the pure BLT phase is obtained in the samples with increased La-content (x = 0.4, 0.5, 0.6, 0.7 and 0.75). These results show that increasing the amount of La reduces the crystallization temperature from 600 °C in BIT to 500 °C in xBLT (with 0.4 ≤ x ≤ 0.75). Sugita et al. [23] recently reported a decrease in the temperature of the formation of the sol-gel derived Bi4−x La x Ti3O12 thin films with increasing La content. The lowest crystallization temperature was 600 °C for the 0.75BLT films and 700 °C for BIT. Therefore, the ethylene glycol-based sol-gel method proves to be more effective in the formation of the BLT phase at comparatively lower temperatures.
Dielectric measurements
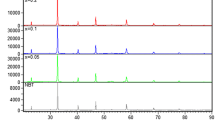
The temperature and frequency dependences of the real part of dielectric permittivity (ɛ′) of the BIT ceramic sintered at 900 °C for 8 h are illustrated in Fig. 3. A dielectric peak appears at ∼355 °C for all the frequencies, but more significant at lower frequencies [Fig. 3(a)]. The 0.1BLT and 0.2BLT ceramics exhibit the same behavior with the peak at 425 and 430 °C, respectively (not shown). This peak is, however, not reproducible, since upon repeating the measurement under the same conditions, it no longer exists. Instead, ɛ′ is found to increase steadily up to the highest measurement temperature of 500 °C with only some changes in the slope [Fig. 3(b)]. The value of ɛ′ reaches 15,000 at 1 kHz. This value is lower than the ɛ′max of ∼40,000 obtained in BIT single crystals at 1 kHz along the a-direction at TC = 675 °C, but higher than the ɛ′max of ∼400 measured along the c-direction [24]. The dielectric loss tangent of BIT ceramic as a function of temperature (upon second run) at various frequencies is presented in Fig. 4(a). It can be seen that the loss increases dramatically with increasing temperature, especially at lower frequencies. Therefore, the high dielectric constant measured at high temperature does not mainly arise from the ionic polarization, but more significantly from the extrinsic contribution of space charges (see below). Thus, it is an apparent value only.
A peak of dielectric permittivity was also observed by several researchers to occur at ∼500 °C in BIT ceramics and single crystals, in addition to the peak associated with T C [8, 24, 25]. This low-temperature maximum was attributed to the space charges generated either in the bulk or at the surface of the crystals [24], and to the ion-jump relaxation [8]. A similar argument can be used to explain our results. It is believed that defects such as oxygen vacancies inherently present in these materials due to the volatilization of Bi–O species create space charges, whose polarization can respond to the external electric field. At low frequencies, these charges have enough time to move longer distances in the sample, creating a larger electronic polarization. Therefore, a high value of (apparent) dielectric constant is measured. At higher frequencies the space charges no longer follow the field, leading to lower values of dielectric constant. With increasing temperature, the effect of charge polarization is enhanced, giving rise to higher (apparent) permittivity values. This explains the strong dispersion observed in both the dielectric constant and loss tangent. At a certain temperature, the space charges are either trapped at the electrode-ferroelectric interface or neutralized. This causes the sudden drop in the permittivity value because the trapped space charges reduce the effective field felt in the bulk of the sample, resulting in poorer polarization response and thereby lower dielectric constant. As the temperature is further increased, more dipoles in the sample are able to switch, leading to an increase in the apparent dielectric constant. The first run of dielectric measurement could therefore act as an annealing process in which defects (and the resulting space charges) are eliminated. As a result, the low-temperature peak is absent in the second measurement [Fig. 3(b)].
The dielectric loss tangent of the 0.2BLT ceramic as a function of temperature at various frequencies is presented in Fig. 4(b). In comparison with the BIT data shown in Fig. 4(a), the loss values at all the frequencies are significantly reduced. Especially, the loss at f > 10 kHz remains lower than the unity up to 500 °C. This improvement of dielectric properties in the xBLT ceramics can be attributed to the effect of La-doping which reduced the oxygen vacancies in the ceramics [12], thereby diminishing the contribution of the space charge polarization. The dielectric constant and loss tangent of the xBLT ceramics measured at 1 kHz as a function of temperature are shown in Fig. 5(a) and (b), respectively. For the xBLT ceramics with x = 0.3, 0.4, 0.5 and 0.6, no peak was observed in the first run of measurement, and the dielectric permittivity increases smoothly from room temperature up to 500 °C. This can also be explained by the effects of La-doping [12], as discussed above. In the 0.7BLT and 0.75BLT ceramics, a dielectric constant peak is observed at T C = 460 and 445 °C, respectively [Fig. 5(a)], which is indicative of the ferroelectric/paraelectric phase transition. This peak also occurs at other frequencies (not shown). For comparison, the T C values for the same compounds were reported in [11] to be 425 and 400 °C, respectively. The higher T C values found in our sol-gel processed ceramics are believed to result from a more stoichiometric composition of the samples which were treated and sintered at relatively low temperature, since it is well known that the loss of Bi–O species can lead to a lowering of T C [21]. From Fig. 5(b), it can be seen that the loss values of the xBLT ceramics are lower than that of BIT in the whole temperature range, confirming the improved dielectric properties by La-doping.
The room temperature dielectric constant and loss tangent of the BIT and xBLT ceramics are listed in Tables 1 and 2, respectively. It can be seen that the 0.5BLT ceramics present a high dielectric constant almost twice the value of BIT, with a reasonably low loss. The 0.7BLT ceramics, on the other hand, show a dielectric constant comparable to BIT, but with a lower loss.
Conclusions
The ceramics of ferroelectric Bi4Ti3O12 [BIT] and Bi4−x La x Ti3O12 [xBLT] (with x = 0.1 − 0.75) of layered perovskite structure have been synthesized by an improved sol-gel process using ethylene glycol as solvent. The effects of soft chemical processing parameters and La-doping on the phase formation and dielectric properties of the BIT and xBLT compounds have been studied. It is shown that, once again, ethylene glycol proves to be a good solvent for the starting materials, and this new sol-gel method is effective in the formation of the pure Bi-layered phase at a temperature as low as 600 °C in BIT and xBLT with x = 0.1, 0.2, and 0.3, and 500 °C in xBLT with \(x = 0.4 - 0.75\), after heat treatment of the gels for 2 h. The sols obtained are stable over a period of 3–5 days, which points to the potential of this sol-gel process for the deposition of the thin films of BIT and xBLT for device applications.
The dielectric constant of the BIT ceramics exhibits a very high value at high temperatures associated with relatively large losses. This high value of apparent dielectric constant, as well as the low temperature peaks in the dielectric permittivity of BIT and xBLT (x = 0.1, and 0.2) can be attributed to the polarization effects of the space charges resulting from such defects as oxygen vacancies. The La-doping is found to improve the dielectric properties of BIT significantly, with a much reduced dielectric loss up to high temperatures and the absence of dielectric permittivity peak below 500 °C in the xBLT ceramics with x ≥ 0.3. The substitution of La for Bi is believed to decrease the oxygen vacancies by reducing the initial Bi2O3 content, resulting in a decrease in space charges and thereby improved dielectric properties. At room temperature, the 0.5BLT ceramics present a dielectric constant much higher than BIT with a reasonably low loss tangent, while the 0.7BLT ceramics show a dielectric constant comparable to BIT, but with a lower loss tangent.
References
J.F. Scott, C.A. Paz de Araujo, Science 246, 1400 (1989)
J.F. Chang, S.B. Desu, J. Mater. Res. 9, 955 (1994)
W.L. Warren, D. Dimos, B.A. Tuttle, R.D. Nasby, G.E. Pike, Appl. Phys. Lett. 65, 1018 (1994)
C.A. Paz de Araujo, J.D. Cuchiaro, L.D. Mcmillan, M.C. Scott, J.F. Scott, Nature (Lond.) 374(13), 627 (1995)
O. Auciello, R. Ramesh, Mater. Res. Bull. 21, 31 (1996)
B. Aurivillius, Ark. Kemi 1, 463 (1949)
J.F. Dorrian, R.E. Newnham, D.K. Smith, M.I. Kay, Ferroelectrics 3, 17–27 (1971)
V.K. Seth, W.A. Schulze, IEEE Trans. Ultrason. Ferroelectr. Freq. Control 36(1), 41–49 (1989)
Y. Ding, J.S. Liu, H.X. Qin, J.S. Zhu, Y.N. Wang, Appl. Phys. Lett. 78, 4175 (2001)
P.C. Joshi, S.B. Krupanidhi, J. Appl. Phys. 72, 5517 (1992)
T. Takenaka, K. Sakata, Ferroelectrics 38, 769–772 (1981)
B.H. Park, B.S. Kang, S.D. Bu, T.W. Noh, J. Lee, W. Jo, Nature (Lond.) 401, 682 (1999)
R.W. Wolfe, R.E. Newnham, J. Electrochem. Soc. 116, 832 (1969)
M. Villegas, A.C. Caballero, C. Moure, P. Duran, J.F. Fernandez, J. Am. Ceram. Soc. 82, 2411 (1999)
A.M. Umabala, M. Suresh, A.V. Prasada Rao, Mater. Lett. 44, 175 (2000)
A.Q. Jiang, H.G. Li, L.D. Zhang, J. Appl. Phys. 83, 4878 (1998)
T. Takeuchi, T. Tani, Y. Saito, Jpn. J. Appl. Phys. 39, 5577 (2000)
P.C. Joshi, A. Mansingh, M. Nkanalasanan, S. Chandra, Appl. Phys. Lett. 59, 2390 (1991)
A.V. Prasada Rao, A.I. Robin, S. Komarnani, Mater. Lett. 28, 469 (1996)
L. Shen, D. Xiao, J. Zhu, P. Yu, J. Zhu, D. Gao, J. Mater. Synth. Process. 9(6), 369 (2002)
K. Babooram, Z.-G. Ye, Chem. Mater. 18, 532 (2006)
A. Hardy, D. Mondelaers, G. Vanhoyland, M.K. Van Bael, J. Mullens, L.C. Van Poucke, J. Sol-Gel Sci. Techn. 26, 1103 (2003)
N. Sugita, M. Osada, E. Tokumitsu, Jpn. J. Appl. Phys. 41, 6810 (2002)
A. Fouskova, L.E. Cross, J. Appl. Phys. 41(7), 2834 (1970)
S. Ehara, K. Muramatsu, M. Shimazu, J. Tanaka, M. Tsukioka, Y. Mori, T. Hattori, H. Tamura, Jpn. J. Appl. Phys. 20(5), 877 (1981)
Acknowledgment
This work was supported by the Natural Science and Engineering Research Council of Canada (NSERC). The authors are grateful to Dr. Y.-H. Bing for help in data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babooram, K., Chin, D.K. & Ye, ZG. Ferroelectric Bi4Ti3O12 and Bi4−x La x Ti3O12 ceramics prepared by a new sol-gel route. J Electroceram 21, 43–48 (2008). https://doi.org/10.1007/s10832-007-9086-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-007-9086-6