Abstract
Effective surface modification of multi-walled carbon nanotubes (MWCNTs) by covering with oleic acid was carried out via the covalent interaction between the hydroxymethylated MWCNTs and the organic long-chained molecules of oleic acid in xylene. The oleic acid modified MWCNTs were characterized through FT-IR spectroscopy, X-ray photoelectron spectrometer, transmission electron microscope, thermogravimetry and dispersion test to confirm that the well dispersed, robust and functional oleic acid had been introduced on the surface of the pristine MWCNTs without sacrificing the original morphological structure obviously. Additionally, the nanocomposites consisting of oleic acid modified MWCNTs and a polystyrene (PS) matrix were fabricated by solution blending method. The structure and morphology of the oleic acid modified MWCNTs/PS nanocompsites were characterized through X-ray diffraction and scanning electron microscopy (SEM). The SEM images showed that the functionalized MWCNTs had a very well dispersion in the matrix. Moreover, the thermal stability and electronic conductivity presented improvements with the filler concentration increased. Meanwhile, the nanocomposites possessed a high dielectric constant with a comparatively low dielectric loss before the MWCNTs concentration up to 1.5 wt%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Owing to the excellent mechanical [1, 2], dielectric [3], electrical [4] and thermal [5] properties and low density, high toughness, and high chemical stability [6] of multi-walled carbon nanotubes (MWCNTs), their polymer-based composites are used as a promising material for numbers applications. Till now, MWCNTs have been used as a structure element in the polymer matrices to obtain functional composites [7]. The composites not only exhibit the excellent properties of organic polymers, but also combine the unique remarkable properties of MWCNTs.
However, the interfacial interaction between MWCNTs and polymer matrix is poor, which is one of issues of nanocomposites. Therefore it is necessary to modify the MWCNTs to improve the dispersion of MWCNTs in polymer matrices [8–10]. Usually, there are mainly two approaches to modify the surface of MWCNTs: (a) the surface modification of inorganic fillers by chemical treatments, (b) grafting functional polymeric molecules to the hydroxyl groups existing on the inorganic fillers [11]. By the two methods, the agglomeration of inorganic fillers due to their large surface area and high surface energy is alleviated, and the dispersion stability of inorganic fillers in polymer matrices can be improved. Most of the previous researches have focused on how to modify the surface of MWCNTs by a variety of approaches. For example, Chen et al. [12] reported styryl group modified MWCNTs showed the better dispersion than pristine MWCNTs. And considerable improvements in mechanical properties and thermal stability of the functionalized MWCNTs/polymer composites were observed.
Recently, the polymer based dielectric composites with the advantages of high dielectric constant, easy processing, high electric breakdown field, lightness, and low cost have received increasing interesting due to their applications in the field of embedded capacitors. In general, there are two strategies to prepare the composite with high dielectric constant. One simple method is introducing ceramic fillers included Ba(Fe0.5Nb0.5)O3, BaTiO3, LiTaO3 and CaCu3Ti4O12 (CCTO) [13–17] with high dielectric constant into polymer matrix. High dielectric constants of several tens have been achieved at around 50 vol% of ceramic powders loading. For example, Yu et al. [18] have recently reported that the dielectric constant of composites with 77 is achieved at 1 kHz after blending 50 vol% treated BT nanoparticles. However, for the composites, the higher dielectric constant over 100 is hard to achieve even if the volume fraction of ceramic particles exceed 50 vol%. And furthermore, the superior properties of the composites described above are spoiled at such a high ceramic powders loading.
Therefore, another attractive strategy has attracted attention. Compared with ceramic/polymer composites, the composites with high dielectric constant could be obtained by adding a small loading of MWCNTs [19–21]. However, normally, MWCNTs/polymer composites are good conductive fillers but not good for dielectric ones due to their high dielectric loss [22], so the introduction of MWCNTs can lead to the dramatic increase in dielectric loss and leakage current, which limits their high frequency applications [23]. In conclusion, it is necessary to modify the MWCNTs and the core–shell MWCNTs have attracted great interest. Because of the existence of the polymer layer at the outer wall of MWCNTs, the compatibility between modified MWCNTs and polymer matrix is improved, and the dielectric loss of the modified MWCNTs could be effectively depressed due to the coating layer. Meanwhile, the inner wall of MWCNTs still remained conductive that led to the high dielectric constant [24, 25].
In the present investigation, the MWCNTs were first treated by nitric acid and formaldehyde solution, and then the surface modification of MWCNTs was conducted by capping with oleic acid [26, 27]. As a result, a covalent bond was formed by the chemical reaction between the hydroxyl groups on the surface of MWCNTs and the carboxyl groups of oleic acid. Finally, the MWCNTs/polystyrene (PS) composites were prepared by solution blending method. The PS was used as the polymer matrix as it containing both better mechanical [28] and dielectric performance [29]. The dispersion of MWCNTs in the polymer matrix, and the thermal, electrical and dielectric properties of MWCNTs/PS composites were discussed.
2 Experimental section
2.1 Materials
Nitric acid, oleic acid, acetone, ethanol, xylene, and tetrahydrofuran were analytically pure reagents (AR). The MWCNTs used in this study was supplied by CNano Technology (Beijing), and PS was purchased from Panjin Ethylene Industrial Company. Deionized water was used throughout the experiments.
2.2 Preparation procedure
2.2.1 Oxidation of MWCNTs
The oxidation of MWCNTs were treated by nitric acid [30]. The procedure was summarized by the following steps: 5 g MWCNTs powders were firstly added into 200 mL concentrated nitric acid (67 %) little by little while stirring and refluxed for 10 h at 100 °C to yield nitric acid oxidized MWCNTs. The oxidized MWCNTs were separated by centrifugation, and then washed using deionized water for several times until the washings showed neutral pH. Finally, the sample was vacuum-dried at 60 °C.
2.2.2 Introduction of hydroxymethyl groups onto MWCNTs surface
The introduction of hydroxymethyl groups onto MWCNTs surface was achieved by treatment of oxidized MWCNTs with formaldehyde solution in alkali conditions. The mixture of MWCNTs and 10 % aqueous solution of HCHO at pH ≈ 10 were stirred at 50 °C for 3 h. After the reaction, the hydroxymethylated MWCNTs was filtered, repeatedly washed to neutral pH, and dried in a vacuum oven at 60 °C for a certain time.
2.2.3 The modification of MWCNTs
Typically, the procedure of the modification of MWCNTs powders by oleic acid was described in the following steps: 3.0 mL of oleic acid and 100 mL of xylene were added into a three-neck flask and vigorously stirred for 0.5 h at 50 °C to form the solution. Then 2.0 g of dried MWCNTs powders was added into the above solution and reacted for 1.5 h at 50 °C. After the reaction, toluene was used to remove the residual impurities, and the precipitate was dried in a vacuum desiccator for 12 h at 80 °C.
The above preparation scheme of oleic acid modified MWCNTS was in Fig. 1.
2.2.4 The preparation of MWCNTs/PS composites
The MWCNTs powders were added into the PS matrix by solution blending, as described in the following steps: PS was dissolved in tetrahydrofuran, and then oleic acid modified MWCNTs powders were added into the PS solution to prepare a series of MWCNTs/PS composites, where n represents the weight fraction of MWCNTs in the PS resins (n = 0.5, 1, 1.5, 2.0, and 3.0 wt%). The mixtures were stirred for 6 h using magnetic stirring at 20 °C. At the conclusion of the reaction, the prepolymers were put into a vacuum oven for 1 h at 65 °C to remove air. Finally, to remove solvent completely and obtain composites, the mixtures were casted into a mold at 60 °C for 24 h.
2.3 Characterization
2.3.1 MWCNTs characterization
In order to check whether the oleic acid has been coated onto the surface of MWCNTs, Fourier-transform-infrared (FT-IR) spectroscopy was carried out using the potassium bromide (KBr) disk method by a Nicolet Avatar 360 Fourier transform infrared spectrometer. The surface composition of the powders was determined by X-ray photoelectron spectroscopy (XPS, Thermo VG ESCALAB 250, Al Kα X-ray source, hv = 1486.6 eV). Transmission electron microscopy (TEM, Hitachi H-600-II, Japan) was conducted at 200 kV to characterize the morphology. For TEM sample preparation, the powders were dispersed in anhydrous ethanol by sonication for 10 min and then fixed on a carbon-coated copper grid (FCF400-Cu).
2.3.2 The properties of MWCNTs/PS composites
The crystalline structure and phase purity of the MWCNTs powders and composites were identified by X-ray diffraction (XRD), using a Bruker D8ADVANCE X-ray diffractometer with a voltage of 40 kV and Cu Kα radiation (λ = 1.5406 Å) in the 2θ range of 10°–80°. The microstructures of the composites were studied using a Tescan 5136MM scanning electron microscope (SEM).
The thermal properties of the composites was characterized by thermogravimetric (TG) analysis on an EXSTAR 6300 instrument in a nitrogen atmosphere in the temperature range of 30–800 °C (with a heating rate of 10 °C/min). The glass transition temperature of the composites was determined using a differential scanning calorimeter (DSC) (Diamond DSC, PerkinElmer Instruments) in a nitrogen atmosphere with a heating rate of 10 °C/min.
The microwave dielectric properties were measured in the frequency range of 1 × 106–1 × 109 Hz using a Novocontrol Broadband Dielectric impedance spectrometer with a HP4191 network analyzer. The circular shaped composites with a 10 mm diameter and a 1 mm thickness were prepared for the dielectric measurement.
3 Results and discussion
3.1 Surface modification of MWCNTs
3.1.1 FT-IR spectra analysis
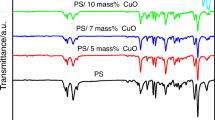
Figure 2 shows the FT-IR spectra of the stages of unmodified and modified MWCNTs. All the samples are dried at 100 °C for 12 h. The FT-IR spectrum of the sample obtained by oleic acid treated revealed some changes in relation to the original sample. Figure 2b shows the FT-IR spectrum of oleic acid modified MWCNTs and besides the characteristic peaks of pristine MWCNTs, new peaks appear. The apparent absorption bonds are observed at 2924 and 2854 cm−1, which are attributed to the asymmetric and symmetric stretching vibrations of the –CH2 group. Furthermore, the stretching vibrations of the C=O group of the oleic acid, which does not appear at the peaks of 1725–1700 cm−1, suggests that a monomolecular layer was formed on the surface of MWCNTs [31].
3.1.2 XPS analysis
XPS is a well-suited method to characterize the surface composition and reveals the local charge density on atoms in solids. Figure 3a shows the XPS spectra of pristine MWCNTs and modified MWCNTs, respectively. The two spectra are similar, but the spectra of modified MWCNTs shows remarkable peaks at about 285.22 and 532 eV, attributing to C and O atoms. It suggests that there is a higher concentration of organic material on the surface of MWCNTs, which can confirm the success of surface treatment. The C1s peaks in XPS spectra of modified MWCNTs (Fig. 3b) were measured to study the concentration of various functional groups. The C1s spectrum of modified MWCNTs mainly consists of a main peak and a shoulder. The main peak at 284.9 eV attributes to sp 2-hybridized graphite-like carbon atoms. Besides, the shoulder is fitted to three types of carbon bonds including C–O (285.6 eV), C=O (286.8 eV), and O–C=O (288.3 eV) [32, 33]. These results demonstrate the success of the modification of MWCNTs by oleic acid after reaction.
3.1.3 TEM analysis
Figure 4 shows the TEM images of the unmodified and oleic acid modified MWCNTs in toluene after sonication (10 min). This result showed that oleic acid does not affect the structure of MWCNTs after modifying. However, with careful observation, it can be seen that modified MWCNTs have larger diameters than the unmodified MCNTs, reflecting the existence of a monomolecular layer on the surfaces of MWCNTs.
3.1.4 TG analysis
In order to calculate the coverage percentage of oleic acid on the surface of MWCNTs, TG analyses were carried out. Figure 5 shows the TG curves of pristine MWCNTs, and oleic acid modified MWCNTs. From curve a, only about 4.54 % weight loss of pristine MWCNTs is found. The TG of oleic acid modified MWCNTs is shown in curve b. The difference in thermal behaviors between pristine and modified MWCNTs is very remarkable. About 3.99 % weight loss of modified MWCNTs at 100 °C, which may be due to the evaporation of water absorbed onto the surface of modified MWCNTs. From 205 to 600 °C, the changing trend of the weight loss of modified MWCNTs is very remarkable. This changing weight loss trend is because of the degradation of coating organic long-chained molecules, of which about 78.83 % of the total weight remains at 600 °C. From two TG curves, the modified particles had 16.63 wt% more weight to lose, which might be the loss of coating organic long-chained molecules.
3.1.5 Dispersion test
Dispersion test is an ideal strategy to check whether a treatment on the surface of MWCNTs has been achieved or not. Figure 6 presents photographs of two vials containing unmodified and modified MWCNTs in PS/THF solutions. All samples were sonicated for 10 min in vials at 20 °C. After 1 month, neither sedimentation nor aggregation of the MWCNTs in the PS matrix is still observed, and there is no obvious stratification, suggesting that the suspension is very stable. In contrast, the unmodified MWCNTs show a relatively faster sedimentation velocity in the solutions during the 2 days. This phenomenon could be explained by the strong interfacial interaction between the modified MWCNTs and the PS matrix.
According to above analyses, it can be concluded that the surface of MWCNTs has been modified by oleic acid without obviously sacrificing the original morphological structure.
3.2 MWCNTs/PS composites
3.2.1 XRD analysis
Figure 7 shows the XRD patterns of pristine MWCNTs and modified MWCNTs, as well as MWCNTs/PS composites. From the XRD patterns of modified MWCNTs, the characteristic peak corresponds to the unmodified MWCNTs. This result showed that oleic acid does not affect the crystalline structure of MWCNTs. However, due to the existence of the oleic acid monomer chains, the intensity of the characteristic peak of the modified MWCNTs was weaker slightly [34]. Furthermore, this characteristic peak of MWCNTs is absent from the XRD patterns of MWCNTs/PS composites, further evidence for efficient mixing of the MWCNTs in PS. After the MWCNTs are incorporated into the PS matrix, the evident reduction in the intensity of the PS becomes more obvious along with the increasing concentration of the MWCNTs, showing that the ordered structure of the PS polymer is damaged more seriously.
3.2.2 SEM analysis
The SEM images of the fractured surfaces of composites containing various weight fractions of MWCNTs are shown in Fig. 8. The bright dots, which are the ends of broken MWCNTs, can be seen everywhere in Fig. 8b with filler concentration was at 0.5 wt% compared with the neat PS in Fig. 8a, which indicated that oleic acid modified MWCNTs were uniformly distributed in PS matrix. However, the MWCNTs network within the polymer is not formed. When the MWCNTs filler concentration was at 1.5 wt%, the network occurred, as shown in Fig. 8c. The MWCNTs network in the composites is essential for good electronic conductivity and remarkable mechanical properties.
3.2.3 TG analysis of composites
To study the thermal stability of the neat PS and the composites, the TG curves of neat PS and MWCNTs/PS composites with different concentrations of MWCNTs are shown in Fig. 9. It is observed that temperature has a negligible effect on the stability of neat PS until it reaches 355 °C, and after this temperature, the neat PS decomposes dramatically. At around 355 °C, the degradation of all the composites does not take place, and the decomposition temperature of the composites is increased. The temperatures of 11.62 wt% weight loss are 333, 386, 389, and 393 °C, respectively for the composites containing 0, 1, 1.5, and 2 wt% of modified MWCNTs powders. It can also be seen that the MWCNTs/PS composites show a better thermal stability than that of the neat PS. This unique decomposition behavior could be attributed to the presence of MWCNTs in PS, because of the good interactions between the PS matrix and the powder, the segmental movement of the PS chains was limited.
3.2.4 DSC analysis of composites
The DSC analyses were carried out to study the glass transition temperatures of the polymer and the composites. Figure 10 shows the thermograms recorded for the neat PS and the composites. It can be seen that the glass transition temperature of neat PS is 96.5 °C. With the increasing of the weight fraction of MWCNTs, the glass transition temperatures of the composites also increase. It can be found that the glass transition temperature of composites with 1.0, 1.5, and 2 wt% are 97.7, 98.6, and 100.3 °C, respectively. In conclusion, the incorporation of MWCNTs increased the glass transition temperatures slightly. This behavior could be explained by the restricting effect of the powders in the polymer. The MWCNTs limited the segmental movement of PS chains, which caused the increase in the glass transition temperature of the composites. High glass transition temperature of composite assures the application in high temperature environments.
According to these results, the thermal stability of MWCNTs/PS composite can be increased by the addition of the MWCNTs.
3.2.5 The electrical properties of composites
MWCNTs have clearly demonstrated their capability as fillers in diverse multifunctional nanocomposites. Figure 11 shows the frequency dependence of AC conductivity for the composites at room temperature. Two distinct behaviors depending on the MWCNTs concentration are observed. For the pristine PS, 0.5, and 1.0 % MWCNTs/PS composites, the AC conductivity increases linearly with the frequency, and it is a typical behavior of insulating materials. When the MWCNTs concentration up to 1.5 wt%, the AC conductivity of the composites has independence of the frequency, which is characteristic for conductive materials. In conclusion, the MWCNTs content when the transition from the insulating to the conducting phase is about 1.5 wt%, which can be attributed to the appearance of the percolation. This result could be explained by the emergence of well-established conductive network formation with 1.5 wt% MWCNTs, which matched the SEM result well.
3.2.6 The dielectric properties of composites
The frequency dependence of dielectric properties for the MWCNTs/PS composites is shown in Fig. 12. From Fig. 12a, it is obvious that the dielectric constant increases with the modified MWCNTs content in the PS resin at all frequencies. The reason is showed as follows: the oleic acid modified MWCNTs will percolate and contact mostly by point-to-point with each other, but the conductive MWCNTs are isolated by the thin oleic acid layer. Such MWCNTs-oleic, acid-MWCNTs contact points are equivalent to nanocapacitors, which results in the high dielectric constant. The dielectric constant of the composite with 3 wt% MWCNTs is 17.8 at 1 × 109 Hz, which is about 7.5 times than the value of 2.37 for the neat PS. As reported in previously studies [24, 35], the dielectric constant of composites dramatically improved with the MWCNTs well dispersed in the polymer. For example, Ning et al. reported that MWCNTs/polyvinyl alcohol (PVA) composite with high dielectric constant was successfully prepared by using electrospinning-in situ film-forming technique. And the dielectric constant was increased from 2.0 at 106 Hz for pure PVA to ≈16 for the composite with 2.0 wt% MWCNTs [35]. The dielectric constants of MWCNTs/PS composites show slight dependence of the frequency before the MWCNTs concentration is up to 1.5 wt%, and the dielectric constant increases remarkably near the percolation threshold of MWCNTs concentration.
Figure 12b illustrates the dielectric loss of the composites in the frequency range of 1 × 106–1 × 109 Hz at room temperature. The results show that the dielectric loss of the composites rises as the modified MWCNTs powder content increases. The reasons of this phenomenon should be described as follows. On one hand, the dielectric properties of the polymer depend on the orientation and relaxation of dipoles, and the process of dipole polarization depends on the movement of polymer chains segments. Therefore, the modified MWCNTs has a strong interaction with the PS matrix, so the orientation and relaxation of dipoles are limited, and the polymer chain segments move more difficultly [36]. On the other hand, due to the existence of the oleic acid layer, a decrease of the electric leakage could occur. Because of the good compatibility between the modified MWCNTs and the PS matrix, it tends to have a bigger restricting influence on the orientation and relaxation of dipoles. Compared with the dielectric loss (0.02865 at 1 × 109 Hz) of neat PS, the value of MWCNTs/PS composite with 1.5 wt% MWCNTs is up to 0.14431 at 1 × 109 Hz. Although MWCNTs can greatly increase the dielectric constant, they also increased the dielectric loss [24, 37]. When the content of MWCNTs are beyond 1.5 wt%, the polymer nanocomposites become conductive materials, and the dielectric loss of the composite is too high to be used as dielectrics due to the large leakage. Thus, the content of MWCNTs plays an important role in enhancing the dielectric constant of the nanocomposites while retaining low dielectric loss.
4 Conclusions
To conclude, nanocomposites consisting of different weight proportion of oleic acid modified MWCNTs and a PS matrix were developed. Firstly, effective surface modification of MWCNTs by covering with oleic acid was carried out via the covalent interaction between the hydroxymethylated MWCNTs and oleic acid in xylene. The FT-IR spectroscopy, XPS, TEM micrographs, TG curves and dispersion test showed that the well dispersed, robust and functional oleic acid had been introduced on the surface of the pristine MWCNTs without sacrificing the original morphological structure obviously. And then the MWCNTs/PS-nanocomposites were fabricated by solution blending method. The investigation of the nanocomposites via XRD and SEM give the evidences for improved interfacial interaction between the oleic acid modified MWCNTs and the PS matrix. The MWCNTs network within the polymer appeared with the filler concentration reached 1.5 wt%, leading to the improvement of electronic conductivity and thermal properties. Finally, the oleic acid modified MWCNTs/PS composites possessed high dielectric constant and comparatively low dielectric loss just before the MWCNTs concentration up to 1.5 wt%.
References
H.Z. Geng, R. Rosen, B. Zheng et al., Adv. Mater. 14, 1387–1390 (2002)
J.N. Coleman, U. Khan, Y.K. Gun’ko, Adv. Mater. 18, 689–706 (2006)
J. Sumfleth, X.C. Adroher, K. Schulte, J. Mater. Sci. 44, 3241–3247 (2009)
J. Sandler, M.S. Shaffer, T. Prasse et al., Polymer 40, 5967–5971 (1999)
X. Lu, G. Xu, J. Appl. Polym. Sci. 65, 2733–2738 (1997)
S. Subramoney, Adv. Mater. 10, 1157–1171 (1998)
Y. Zhang, Z. Li, H. Li et al., J. Mater. Sci. Mater. Electron. 25, 2692–2696 (2014)
B. Fiedler, F.H. Gojny, M.H. Wichmann et al., Compos. Sci. Technol. 66, 3115–3125 (2006)
F. Jin, M. Feng, K. Jia, X. Liu, J. Mater. Sci. Mater. Electron. 26, 5152–5160 (2015)
J. Zhong, H. Tang, Y. Chen, X. Liu, J. Mater. Sci. Mater. Electron. 21, 1244–1248 (2010)
D. Tasis, N. Tagmatarchis, A. Bianco et al., Chem. Rev. 106, 1105–1136 (2006)
X. Chen, F. Tao et al., Mater. Sci. Eng. 499, 469–475 (2009)
P.K. Patel, K.L. Yadav, H. Singh et al., J. Alloys Compd. 591, 224–229 (2014)
S. Wu, S. Wang, L. Chen et al., J. Mater. Sci. Mater. Electron. 19, 505–508 (2008)
L.P. Curecheriu, L. Mitoseriu, A. Ianculescu, J. Alloys Compd. 482, 1–4 (2009)
P. Guggilla, A.K. Batra, M.E. Edwards, J. Mater. Sci. 44, 5469–5474 (2009)
E. Swatsitang, A. Niyompan, T. Putjuso, J. Mater. Sci. Mater. Electron. 24, 3514–3520 (2013)
K. Yu, Y. Niu, Y. Zhou et al., J. Am. Ceram. Soc. 96, 2519–2524 (2013)
A. Peigney, C. Laurent, E. Flahaut et al., Ceram. Int. 26, 677–683 (2000)
Y. Shen, G. Liang, L. Yuan et al., J. Alloys Compd. 602, 16–25 (2014)
M.H. Al-Saleh, W.H. Saadeh, U. Sundararaj, Carbon 60, 146–156 (2013)
M.A. Rahman, G.S. Chung, J. Alloys Compd. 581, 724–730 (2013)
Z.M. Dang, L. Wang, Y.I. Yin et al., Adv. Mater. 19, 852–857 (2007)
C. Yang, Y. Lin, C.W. Nan, Carbon 47, 1096–1101 (2009)
H. Liu, Y. Shen, Y. Song et al., Adv. Mater. 23, 5104–5108 (2011)
Q. Shu, Q. Zhang, G. Xu et al., Food Bioprod. Process. 87, 164–170 (2009)
S.V. Mahajan, S.A. Hasan, J. Cho, Nanotechnology 19, 195301 (2008)
H.S. Vaziri, M. Abadyan, M. Nouri et al., J. Mater. Sci. 46, 5628–5638 (2011)
M. Rabuffi, G. Picci, IEEE Trans. Plasma Sci. 30, 1939–1942 (2002)
I.D. Rosca, F. Watari, M. Uo et al., Carbon 43, 3124–3131 (2005)
R.Y. Hong, J.Z. Qian, J.X. Cao, Powder Technol. 163, 160–168 (2006)
H. Wu, A. Gu, G. Liang et al., J. Mater. Chem. 21, 14838–14848 (2011)
V. Simon, M. Todea, A.F. Takács et al., Solid State Commun. 141, 42–47 (2007)
J. Deng, X. Ding, W. Zhang et al., Eur. Polym. J. 38, 2497–2501 (2002)
N. Ning, X. Bai, D. Yang et al., RSC Adv. 4, 4543–4551 (2014)
R. Gregorio Jr, E.M. Ueno, J. Mater. Sci. 34, 4489–4500 (1999)
Y. Li, C. Chen, J.T. Li, S. Zhang et al., Nanoscale Res. Lett. 5, 1170–1176 (2010)
Acknowledgments
The project was supported by the National Natural Science Foundation of China (NSFC, No. 21246002), National Post-doctoral Science Foundation, Technology Innovation Foundation of MOST (No. 11C26223204581), Natural Science Foundation of Jiangsu Prov. (No. BK2011328), 333 Talent Project (2013) of Jiangsu Prov., the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Minjiang Scholarship of Fujian Prov.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L.T., Chen, Q., Hong, R.Y. et al. Preparation of oleic acid modified multi-walled carbon nanotubes for polystyrene matrix and enhanced properties by solution blending. J Mater Sci: Mater Electron 26, 8667–8675 (2015). https://doi.org/10.1007/s10854-015-3542-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3542-x