Abstract
The effect of the nature of solvent on polypyrrole (PPy) synthesis by in situ coating on the prior synthesized carbon coated LiFePO4 for electrochemical properties of composite materials designated as LiFePO4/C-PPy has been investigated. An in situ chemical oxidation method is used for the polymerization of pyrrole by using three different solvents e.g. water, ethanol and acetonitrile (ACN). The structure and morphology of PPy grown as coating is characterized by Raman spectroscopy and scanning electron microscopy respectively. X-ray diffraction patterns of the composites confirm the presence of Li0.05FePO4 compound as a minor phase along with major LiFePO4 phase. The ACN assisted synthesized PPy coating shows best electrochemical performance as cathode material among selected solvents used for the synthesis. Electrochemical impedance spectroscopy indicates lowest electrode impedance and highest Li-ion diffusion for the composite LiFePO4/C-PPy synthesized in ACN solvent as compared to the water and ethanol derived samples. The higher rate capability (82 mAh g−1) of the composite LiFePO4/C-PPy synthesized in ACN solvent as compared to LiFePO4/C (42 mAh g−1), is attributed to the morphology and structure of the polymer coating.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The use of LiFePO4 as cathode material for rechargeable lithium ion batteries has been increasing in many applications like small scale electronic devices to electric vehicle (EV) or hybrid electric vehicle. The LiFePO4 cathode material has several advantages such as they are non-toxic, inexpensive, environment friendly and have flat discharge voltage at 3.4 V versus Li/Li+ and good theoretical charge capacity of 170 mAh g−1 [1, 2]. But the material LiFePO4 does not provide good rate capability as it has poor electronic conductivity (~10−9 S cm−1) and low ionic diffusivity (10−17–10−14 cm2 s−1) [3]. Many efforts have been made by the researchers to improve the electronic conductivity and ionic diffusivity of the LiFePO4 material. Surface coating and doping of the oxide particles are proved to be effective to achieve good conductivity of the material [4, 5]. Carbon coating has been used to improve the conductivity of LiFePO4, but one of the major drawbacks is that the carbon is electrochemically inactive and therefore leads to decrease the volumetric energy density of the electrode [6]. Conducting polymer coating around LiFePO4 particles have improved rate capability of LiFePO4 [7].
PPy shows the electronic conductivity due to delocalization of pie-electrons in the conjugated pie-bond system [8]. The conductivity of the synthesized PPy can further be improved by using suitable solvent and doping with organic dopant sodium p-toluene sulfonate (Na-PTSA) or inorganic dopants ClO −4 , BF −4 and NO −3 [8, 9]. The doping with Na-PTSA as compared to inorganic dopants results in good and stable electronic conductivity of PPy [10].
In the present study, PPy is synthesized using different solvents viz. water, ethanol and ACN. The study has indicated that ACN solvent derived PPy coating on LiFePO4/C has highest specific capacity. The polarity of solvent used for the polymerization affects the morphology of PPy [11].
2 Experimental
2.1 Synthesis of carbon coated LiFePO4
The synthesis of carbon coated LiFePO4 (designated as LiFePO4/C) was carried out in two steps. In the first step, 1.0 ml of aniline monomer (99.5 %, GR, Merck) and 2.62 g of (NH4)H2(PO4) (99 %, GR, Merck) are mixed in 200 ml of Millipore water. A dropwise addition of 100 ml of aqueous solution of 3.7 g of FeCl3 (98 %, Merck) was made into the previous mixture. White colored FePO4 precipitate forms instantaneously in the solution. The solution which initially was white in color slowly becomes light green because of the formation of polyaniline on the surface of FePO4 particles 4 h after the addition of aqueous solution of FeCl3. In the second step, the synthesized material in the powder form was ground in a pestle-mortar with Li(CH3COO) (99 %, Loba chemie.) powder maintaining molar ratio of 1:1 between Li and Fe. The mixture was heated at 400 °C for 4 h under the Ar-10 % H2 gas atmosphere before calcination and was further grinded in a pestle-mortar for 30 min to homogenize the mixture. The material was again heated at 700 °C for 15 h under Ar-10 % H2 atmosphere for calcination. The calcined material was further washed thoroughly in deionized water and acetone to remove chlorine to get pure LiFePO4/C powder.
2.2 Synthesis of PPy
For synthesizing PPy, three suspensions each were containing 1 ml of pyrrole monomer in 40 ml of each solvent (water, ethanol and ACN) were made separately and stirred at 0–5 °C. In each suspension 0.95 g of Na-PTSA was added. Further 7.25 g of FeCl3 dissolved in 20 ml of corresponding solvent was added dropwise in the above suspensions prepared. The color of the solution changes from transparent to black on the addition of FeCl3 solution showing the formation of PPy precipitates inside the suspension. The molar ratio of the additive Na-PTSA to pyrrole was 1:3 and oxidizing agent to pyrrole was kept at 3:1 [12]. The reaction was carried out for 4 h with continuous stirring. After 4 h the solution was filtered and washed with their respective solvents used. The filtered material was dried in vacuum oven for 12 h at 60 °C.
2.3 Synthesis of LiFePO4/C-PPy composite
For the synthesis of LiFePO4/C-PPy composites, 1 g of LiFePO4/C powder added in 40 ml solvent each of water, ethanol and ACN were sonicated for 15 min to prepare the suspensions. 1 ml of pyrrole monomer was added in each suspension under continuous stirring between 0 and 5 °C. After homogenization for 15 min with continuous stirring, 0.95 g of Na-PTSA was added in the above suspensions followed by the addition of FeCl3 (7.25 g) dissolved in corresponding solvents to each suspension. After 4 h of polymerization the solutions were filtered and washed with the corresponding solvents. The synthesized materials were dried in vacuum oven for 12 h at 60 °C to get the LiFePO4/C-PPy composite. The LiFePO4/C-PPy composite materials synthesized in each of ACN, ethanol and water solvents were designated as LiFePO4/C-PPy(ACN), LiFePO4/C-PPy(ethanol) and LiFePO4/C-PPy(water).
2.4 Physical characterizations
XRD patterns of the LiFePO4 and LiFePO4/C-PPy composites were obtained using a Bruker (AXS, D8 advance) X-ray diffractometer using X-ray radiation (λCuKα = 1.5406 Å) with a scanning rate of 1.5 º/min. The morphologies of samples were observed by field emission transmission electron microscope (TEM, FEI-TECHNAI-20) and field emission scanning electron microscope (SEM, FEI Quanta 200F). The PPy contents in the samples were estimated by thermogravimetric analysis of the samples using thermal analyzer (Seiko Instruments, EXSTAR TG/DTA 6300). Raman confocal micro-spectrometer system (inVia, Renishaw) was used to characterize the carbon coating on the samples for its graphitization and confirming the formation of PPy in the LiFePO4/C-PPy composites. He–Ne LASER beam having wavelength of 514 nm as the excitation source was used to obtain Raman spectra of the synthesized materials.
2.5 Electrochemical characterization
The slurries were prepared with active material, acetylene black, and PVDF binder in the proportion (in weight %) of 70:15:15 respectively mixed in the NMP solvent. For preparation of the electrode aluminum foil was coated with the slurry. The coating was dried under vacuum at 120 °C for 24 h. The dried coating was pressed using a hydraulic press at a pressure of 500 kN m−2 to ensure good adherence of the paste material with the aluminium foil. The coated foils were cut into the required size of the working electrode. The counter electrode of lithium metal and working electrode were assembled in a laboratory fabricated Teflon cell inside the glove box under high purity argon atmosphere having controlled moisture and oxygen contents of <0.1 ppm. The complete Teflon cell assembly consists of a cathode (working electrode), an anode (lithium metal foil), a polypropylene microporous separator membrane soaked with a liquid electrolyte of 1 mol L−1 LiPF6 dissolved in a 1:1 v/v solution of dimethyl carbonate (DMC) and ethylene carbonate (EC). The electrochemical and cyclic voltammetry tests were performed using a battery testing system (BT2000, Arbin Instruments). The cell was charged and discharged galvanostatically over the voltage range between 2.2 and 4.2 V (vs. Li/Li+) at different current rates. Electrochemical impedance spectroscopy (EIS) analyses of the cells were carried out on the electrochemical workstation (Gamry Instruments) in the frequency range from 0.1 Hz to 100 kHz and at a voltage of 0.5 mV.
3 Results and discussions
3.1 XRD-analysis
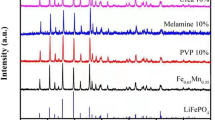
Figure 1 shows the XRD-patterns of LiFePO4/C and LiFePO4/C-PPy composite materials. All the diffraction peaks for the sample LiFePO4/C can be indexed according to the diffraction pattern of LiFePO4 having olivine-orthorhombic structure (JCPDS card No. 01-083-2092) and space group Pnma (62). The diffraction pattern of the composite LiFePO4/C-PPy shows that material consists of two phases of LiFePO4 (JCPDS file No. 01-083-2092) and Li0.05FePO4 (JCPDS file No. 98-10-8284). The formation of Li0.05FePO4 phase implies that lithium depletion has taken place due to the lithium extraction by the polymer coating of PPy because the polymer is electrochemically active. Both the phases of LiFePO4 and Li0.05FePO4 are reported to have same capacity [13]. The characteristic peaks of carbon and PPy are not visible in the XRD patterns (Fig. 1) which imply that the PPy coating is amorphous and carbon content in the materials is sufficiently low. Moreover, a fraction of the carbon is of disordered form that is confirmed by Raman spectroscopic analysis of the sample.
The Table 1 shows the major peak positions and intensities of Li0.05FePO4 (JCPDS file No. 98-10-8284) and LiFePO4 (JCPDS file No. 01-083-2092). The resolved and unresolved peaks of Li0.05FePO4 in the observed XRD pattern are also indicated.
3.2 Thermal analysis
Figure 2 shows the TG profiles of the samples LiFePO4/C-PPy(water), LiFePO4/C-PPy(ethanol), LiFePO4/C-PPy(ACN) and pristine PPy heated at the rate of 10 °C min−1 from room temperature to 800 °C at which the materials were held for 1 h to remove the PPy content completely by decomposition. A nominal weight loss was occurred over the temperature range up to 120 °C in all the samples because of the removal of water molecules from the samples. Above 120 °C, a gradual weight loss of the materials take place up to 800 °C due to decomposition of PPy coating. The steep fall in the weight at 800 °C is due to decomposition of PPy at that temperature. The polymer content in LiFePO4/C-PPy(water), LiFePO4/C-PPy(ethanol), LiFePO4/C-PPy(ACN) were estimated as 6.9, 5.5 and 6.6 wt% respectively.
3.3 Morphology of LiFePO4/C-PPy
SEM micrographs of the pristine PPy, LiFePO4/C and LiFePO4/C-PPy composites are shown in Fig. 3. The Fig. 3a, b show the morphologies of pristine PPy synthesized in ACN and water respectively. The PPy powder prepared in ACN solvent has low porosity and smooth surface than that of powder synthesized in water. The polymerization process involves the formation of radical cation of pyrrole due to its oxidation by FeCl3.
During polymerization the Pyrrole radical cation combines with other similar radical cation for chain propagation of the polymer. But if the polymer synthesis is processed in water or ethanol the active centers of the growing chain were attacked by the –OH and C = O nucleophilic groups instead of other free pyrrole radical [14]. Due to the attack of –OH and C = O, PPy chains become hydrophobic to the solvent resulting in the lowering of active centers and formation of aggregates of PPy. So, the PPy synthesized in the water or ethanol solvents have more porosity and surface roughness as compared to the ACN derived PPy [15].
The FESEM micrograph of LiFePO4/C particles (Fig. 3f) shows that particles are nearly spherical having size range of 100–500 nm. The LiFePO4/C particles are agglomerated through the carbon present on the surface of LiFePO4 particles. Figure 3c–e show the SEM micrographs of the composites LiFePO4/C-PPy(ACN), LiFePO4/C-PPy (water) and LiFePO4/C-PPy (ethanol) respectively. The micrographs show that the morphology of the PPy is same as that of pristine PPy synthesized in the corresponding solvents. The PPy in the composites LiFePO4/C-PPy (water) and LiFePO4/C-PPy (ethanol) partially make the coverage of the underneath materials component. It is also observed that the PPy chains grow separately in the coating region. The surface roughness and porosity of the PPy coating is higher in the LiFePO4/C-PPy(water) and LiFePO4/C-PPy(ethanol) as compared to that of LiFePO4/C-PPy(ACN). The micrograph in Fig. 3c suggests that LiFePO4/C-PPy(ACN) composite is largely covered by the PPy.
3.4 TEM analysis
Figure 4a, c show the TEM micrographs of LiFePO4/C and LiFePO4/C-PPy(ACN) composite powder materials. Figure 4b presents a high resolution micrograph of Fig. 4a to show the lattice fringes of the particle and gives the carbon coating thickness which was estimated to be 3 nm. The surface coverage of the LiFePO4/C particles with the PPy is observed in the micrograph of Fig. 4c. Though the particles are seen present in the agglomerate state, however, the regular (nearly spherical) shape of the particles remains unchanged after surface coating with the polymer. An average size of all the LiFePO4/C-PPy composites particle is ~300 nm.
3.5 Raman spectra analysis
The structure of carbon and PPy coatings that developed on the LiFePO4 particles is very important for assessing the electronic conductivity and Li-ion diffusivity of the LiFePO4/C material. Raman analysis was performed to know the structure of carbon and PPy. The broad and high intensity bands at 1350 and 1580 cm−1 in Raman spectra of LiFePO4/C signify the D and G-bands of carbon (Fig. 5). The D-band originates because of defects associated with finite graphene size such as edges with special shapes and atomic defect within a graphene layer, leading to break of symmetry in the structure [16]. The A 1g mode of vibration, occurs at the k1 (edge) point of first Brillion zone that give rise to D-band. For the infinite graphitic size the change in polarizability is cancelled, therefore A 1g mode of vibration is Raman inactive. But for finite graphitic size polarizability change exists due to which graphitic carbon become, Raman active. So, the intensity at 1350 cm−1 was attributed to A 1g mode for finite size of graphitic carbon or at the edges of lager graphitic carbon [17]. While the G-band represents highly ordered graphitic carbon. Li et al. [18] have reported that the small intensity ratio (R = ID/IG) of the D-band and G-band indicates higher fraction of graphitic carbon and hence higher conductivity. The Fig. 5 shows the Gaussian fittings of the Raman spectra with de-convolution of D-band and G-band for LiFePO4/C and LiFePO4/C-PPy composites to measure the R values. The values of R for LiFePO4/C, LiFePO4/C-PPy(water), LiFePO4/C-PPy (ethanol) and LiFePO4/C-PPy (ACN) are 0.97, 0.77, 0.74 and 0.67 respectively. The decrease in R values for all LiFePO4/C-PPy composites shows improved graphitic carbon as compared to LiFePO4/C. This is attributed to formation of H-bonds as a result of interaction between PPy and defect sites of carbon [19]. H-bonding occurs when nearly spherical shape ceramic particles were coated with polymer chains [20]. A minimum value (0.67) of R has been obtained for LiFePO4/C-PPy(ACN). This is because polypyrrole coating of minimum porosity grown in ACN solvent offers highest contact area with carbon coating. Hence large number of defects was interacted with PPy coating resulting decreased D-band intensity for LiFePO4/C-PPy(ACN) composite.
The growth of PPy was confirmed by the Raman spectra analysis (Fig. 5) of LiFePO4/C-PPy composites. Raman spectrum of LiFePO4/C-PPy(water) composite has weak bands at 995 and 1043 cm−1. The band at 995 cm−1 is associated with the ring deformation vibration which has a consequence of polaron formation. The low intensity peak at 1043 cm−1 is assigned to symmetric C–H in-plane bending vibration in the benzoid ring [21]. The peaks at 935 and 937 cm−1 for the composites LiFePO4/C-PPy(ACN) and LiFePO4/C-PPy(ethanol) respectively arise due to C–H out of plane deformation in the quinoid ring and represent the bipolaron state of PPy [22]. The peak located at 1070 cm−1 for LiFePO4/C-PPy(ACN) arise due to the C–H in-plane bending vibration occurred in the oxidized state of PPy. Although polaron and bipolaron states give rise to the conductivity of PPy, but bipolaron state favors the higher conductivity as compared to the polaron state [23]. Even though a single bipolaron peak at 935 cm−1 was observed for LiFePO4/C-PPy(ethanol), but the detection of two peaks at 935 and 1070 cm−1 reflects the higher oxidation of PPy in LiFePO4/C-PPy(ACN) which supports the higher conductivity.
3.6 EIS testing
EIS analyses were made to investigate the effect of solvents on the conductivity of the LiFePO4/C and all LiFePO4/C-PPy electrode materials. Figure 6a shows the EIS profiles of LiFePO4/C and LiFePO4/C-PPy composite electrodes after five cycles of charging and discharging at 1 C rate. The measurements were performed at room temperature over the test frequencies between 0.1 Hz and 100 kHz. The EIS profiles can be explained with the help of equivalent circuit as shown in the inset of Fig. 6a. The equivalent circuit has a resistance (Rs) in series with a parallel combination of double-layer capacitance (Cdl), and charge transfer resistance (Rct) in series with Warburg impedance (Zw) [24]. Intercept of the semi-circle with Zreal in the high frequency region represents the ohmic resistance (Rs) which corresponds to interfacial resistance between the electrolyte and electrode. The intercept of semi-circle in the intermediate frequency range represents the charge transfer resistance (Rct). A double layer capacitance was introduced to represent the constant phase element. The line in the low frequency region represents the Warburg impedance (Zw) which corresponds to the Li-ion diffusion in the LiFePO4 particles [25].
The low frequency response of EIS corresponds to the transport of Li-ion and electron into the electrode material [26]. So the diffusivity of Li-ion has been widely reported in the low frequency region of EIS using the expression [26, 27].
Herein R is gas constant (8.314 J mol−1K−1), T is absolute temperature (300 K) of electrode during measurement, A is area of cathode–electrolyte interface, n is the number of electrons per molecule during oxidization (for LiFePO4, n = 1), F is the Faraday constant (96,487 C mol−1), c is the molar concentration of lithium ion (1.57 × 10−2 mol cm−3), σ ω is the Warburg factor.
Warburg factor can be calculated from the following relation:
The slope of the plot Z real versus w −1/2 in Fig. 6b in the low frequency region yields the σ ω where ω is the angular frequency of the applied alternating current. The slopes of the linear fitting of the points in the low frequency region have been listed in the Table 2 for each sample. Using the relations (3) and (4), the calculated values of Li-ion diffusion coefficient are 7.37 × 10−15, 7.56 × 10−14, 4.00 × 10−13 and 6.23 × 10−13 cm2 s−1 for the samples LiFePO4/C, LiFePO4/C-PPy(water), LiFePO4-PPy(ethanol) and LiFePO4/C-PPy(ACN) respectively at room temperature. The calculated values of diffusivity show improvement as compared to 7.56 × 10−14 and 1.26 × 10−14 cm2 s−1 reported in the literature for LiFePO4/C composite [28, 29]. The highest Li-ion diffusivity for LiFePO4/C-PPy(ACN) composite can be explained by the Raman analysis. The interaction between PPy and defect sites of carbon coating reduces the interaction of Li-ion with the defect sites. The reduced interaction facilitates smooth movement of Li-ion on the graphitic carbon resulting higher diffusivity to the LiFePO4/C-PPy(ACN) material [30].
The exchange current density is a factor which also reflects the electrochemical reversibility of the electrode and can be obtained from the following relation [31].
For all the synthesized materials, values of i 0 are calculated from the relation (5) and listed in the Table 2. The composite LiFePO4/C-PPy(ACN) has the highest exchange current density 1.20 × 10−4 mA cm−2 of all the composite materials studied, reflecting best electrochemical reversibility. The improved exchange current density of 1.01 × 10−4 and 1.20 × 10−4 mA cm−2 was reported by ammonia assisted LiFePO4/C and Na–Ti doping in LiFePO4/C respectively [31, 32]. The minimum R ct value was obtained for LiFePO4/C-PPy(ACN) and consequently electronic conductivity of this composite material is highest among all the composites prepared.
The higher electronic conductivity of the sample LiFePO4/C-PPy(ACN) over samples LiFePO4/C-PPy(water) and LiFePO4/C-PPy(ethanol) was attributed to the following effects. (1) The bipolaron formation in PPy coating in ACN solvent as compared to polaron formation in water. (2) Generation of –OH and C = O groups during polymerization of pyrrole in water and ethanol solvents restrict the delocalization of polaron and bipolaron [15]. (3) The generated –OH and C = O groups during the synthesis attack on the active sites which restricts the PPy chain growth mechanism and resulting in the higher porosity [15]. On the other hand, higher porosity generated during the synthesis of PPy produce the discontinuity in the surface coating and hence larger path length for the conducting species. As a result Fig. 6a shows LiFePO4/C-PPy(ACN) composite giving minimum Rct = 215.6 Ω as compared to 500.3, 368.8 and 252.0 Ω for samples LiFePO4/C, LiFePO4/C-PPy(water) and LiFePO4/C-PPy(ethanol) respectively.
3.7 Electrochemical analysis
The discharge profiles of the cathode materials LiFePO4/C-PPy(ACN), LiFePO4/C-PPy(ethanol), and LiFePO4/C-PPy(water) measured at 0.2, 0.5, 1, 2, 5, 10 and 20 C-rates are presented in a manner comparative with that of LiFePO4/C, in Fig. 7a–c. It is seen that the rate capability has enhanced for all the PPy coated composites as compared to LiFePO4/C. For LiFePO4/C-PPy(ACN), LiFePO4/C-PPy(ethanol), LiFePO4/C-PPy(water) and LiFePO4/C materials the discharge capacities are 139, 137, 138 and 142 mAh g−1 respectively obtained at low C-rate (0.2 C), and 82, 60, 54 and 42 mAh g−1 respectively at high C-rate (20 C). The measurements at 5, 10 and 20 C-rates for the composite LiFePO4/C-PPy(ACN) show discharge capacities of 98, 91 and 82 mAh g−1 respectively. However the discharge capacities of LiFePO4/C-PPy(ethanol) at 5, 10 and 20 C-rates are 83, 71 and 60 mAh g−1 respectively. The enhanced rate capability of LiFePO4/C-PPy composite material synthesized electrochemically was reported by Huang et al. [33]. All the LiFePO4/C-PPy composites show a minor decrease in discharge capacity at low current rates. At low current rates the discharge capacities of LiFePO4/C-PPy and LiFePO4/C are comparable as PPy coating do not have significant effect on the conduction because of slow movement of charge species. However the difference in capacities has substantially increased when current is increased up to 20 C. From Fig. 7a–c one can infer that LiFePO4/C-PPy(ACN) has higher rate capability than other counter parts synthesized in water and ethanol solvents. In order to investigate the higher capacity of LiFePO4/C-PPy(ACN) conductivity measurements of all the samples were made and the results are consistent with the electrochemical measurements. The low electronic conductivity of the LiFePO4/C-PPy(water) and LiFePO4/C-PPy(ethanol), and improved Li-ion diffusion in the LiFePO4/C-PPy(ACN) composite was supported by morphological and structural analysis of the PPy coating.
It has been reported in many studies [30, 34] that higher number of defects on carbon coating offers more sites to occupy Li-ions, but there may be no de-intercalation from these sites due to which less number of Li-ions participate in the charging/discharging process, resulting increased irreversible capacity. The Raman analysis confirmed the minimum number of defects on the carbon for LiFePO4/C-PPy(ACN) which may be occupied by the Li-ions and contribute to the lowest irreversible capacity followed by LiFePO4/C-PPy(ethanol) and LiFePO4/C-PPy(water). The Fig. 7d compares the discharge capacities for all the LiFePO4/C-PPy(ACN), LiFePO4/C-PPy(ethanol) and LiFePO4/C-PPy(water) composites at typically 2 C-rate for 50 cycles. All the LiFePO4/C-PPy samples shows superior cyclability in comparison with LiFePO4/C. The capacity fade of 2, 5, 9 11 % as compared to initial capacity were observed for LiFePO4/C-PPy(ACN), LiFePO4/C-PPy(ethanol), LiFePO4/C-PPy(water) and LiFePO4/C samples respectively over 50 cycles. This shows that PPy addition to LiFePO4/C improves the cyclability and rate capability for all the solvents.
Figure 8a shows the discharge capacities of the materials measured at different C-rates and for every C-rate, measurements were made for five cycles. In order to investigate the rate capability of composites LiFePO4/C-PPy(ACN), LiFePO4/C-PPy(ethanol) and LiFePO4/C-PPy(water), a factor ‘differential capacity (DC)’ as expressed below, has been devised.
The Fig. 8b represents the plot of differential capacity (DC) versus C-rate. From the graph it can be inferred that the DC values are very close to zero, at lower discharging rates of 0.2 and 0.5 C respectively. At moderate discharge rates of 1, 2 and 5 C the DC of each samples start spreading and shows maximum spread at highest 20 C rate. The sample LiFePO4/C-PPy(water) have a slight dip in DC at 10 C rate showing a decrease in the rate capability as compared to 5 C. The highest DC value of 1.08 have been obtained for LiFePO4/C-PPy(ACN) composite material at 20 C rate implying that it is a high rate capability material followed by LiFePO4/C-PPy(Ethanol) and LiFePO4/C-PPy(Ethanol).
Figure 9a shows the discharge capacity of pure PPy synthesized in water, ethanol and ACN solvents, relative to cell voltage. The profile of graph shows that the PPy samples are electrochemically active in the range of 4.2–2.0 V, delivering the specific discharge capacities of 61, 64 and 68 mAh g−1 for water, ethanol and ACN solvents respectively at 1 C rate. The discharge capacity of PPy is highest for ACN solvent followed by those prepared in ethanol and water. This was due to the fact that electronic conductivity and Li-ion diffusivity were affected by the solvent and morphology of PPy. The continuous nature of PPy coating derived in ACN solvent is the reason for higher capacity of the composite. In both coating and bulk, PPy synthesized in ACN has denser structure as compared to that prepared in water and ethanol solvents. The discontinuity (Fig. 2b) restricts the flow of conducting species due to which the overall conductivity of material decrease [15]. So, a continuous coating is required to achieve good conductivity of the material. The discharge capacity of the polymers synthesized in different solvents with respect to cycle number is given in the Fig. 7b for 20 cycles. The discharge capacities of PPy synthesized in ACN is almost constant. However, water and ethanol solvents deliver 92 and 96 % of the initial capacity respectively, after 20 cycles, showing the maximum stability of PPy synthesized in ACN.
4 Conclusions
The material LiFePO4/C has been prepared by chemical precipitation method followed by calcinations in the presence of Ar-10 %H2 atmosphere. The polypyrrole coating on the surface of LiFePO4/C has been produced by chemical oxidation method using FeCl3 as oxidizing agent in different solvents viz. water, ethanol, and ACN. The composite LiFePO4/C-PPy(ACN) produces appreciable rate capability of 82 mAh g−1 at 20 C. The superior electrochemical performance of the LiFePO4/C-PPy(ACN) is attributed to the better electronic conductivity and enhanced Li-ion diffusion. The composite LiFePO4/C-PPy(ACN) shows better electronic conductivity due to the bipolaron formation within the PPy chains. The formation of –OH and C = O groups during polymerization depreciate conductivity for samples LiFePO4/C-PPy(water) and LiFePO4/C-PPy(ethanol) on the other hand the formation of bipolaron supports the higher electronic conductivity for LiFePO4/C-PPy(ACN). The improved Li-ion diffusion for LiFePO4/C-PPy(ACN) was attributed to the reduced interaction offered by the decreased number of defect sites on carbon coating.
References
C.W. Ong, Y.K. Lin, J.S. Chen, J. Electrochem. Soc. 154, A527 (2007)
S.Y. Chung, J.T. Bloking, Y.M. Chiang, Nat. Mater. 1, 123 (2002)
B. Jin, H.B. Gu, W. Zhang, K.H. Park, G. Sun, J. Solid State Electrochem. 12, 1549 (2008)
Z. Yang, J. Xia, L. Zhi, W. Zhang, B. Pei, Ionics 20, 169 (2014)
Y. Chen, J. Mao, J. Mater. Sci. Mater. Electron. 25, 5153 (2014)
Z. Chena, J.R. Dahna, J. Electrochem. Soc. 149, A1184 (2002)
Z. Liu, S.W. Tay, L. Hong, J.Y. Lee, J. Solid State Electrochem. 15, 205 (2011)
A.K. Wanekaya, Y. Lei, E. Bekyarova, W. Chen, R. Haddon, A. Mulchandani, N.V. Myung, Electroanalysis 18, 1047 (2006)
S. Machida, S. Miyata, Synth. Met. 31, 311 (1989)
A. Kaynak, Fibers. Polym. 10, 590 (2009)
K.A. Noh, D.W. Kim, C.S. Jin, K.H. Shin, J.H. Kim, J.M. Ko, J. Power Sources 124, 593 (2003)
L. Yuan, J. Wang, S.Y. Chew, J. Chen, Z.P. Guo, L. Zhao, K. Konstantinov, H.K. Liu, J. Power Sources 174, 1183 (2007)
A. Yamada, H. Koizumi, S.I. Nishimura, N. Sonoyama, R. Kanno, M. Yonemura, T. Nakamura, Y. Kobayashi, Nat. Mater. 5, 357 (2006)
I. Rodrıguez, B.R. Scharifker, J. Mostany, J. Electroanal. Chem. 491, 117 (2000)
J.M. Ko, H.W. Rhee, S.M. Park, C.Y. Kim, J. Electrochem. Soc. 137, 905 (1990)
M.S. Dresselhaus, A. Jorio, R. Saito, Annu. Rev. Condens. Matter Phys. 1, 89 (2010)
F. Tuinstra, J.L. Koenig, J. Chem. Phys. 53, 1126 (1970)
J. Li, Q. Qu, L. Zhang, L. Zhang, H. Zheng, J. Alloy Compd. 579, 377 (2013)
M.L. Singla, R. Sehrawat, N. Rana, K. Singh, J. Nanopart. Res. 13, 2109 (2011)
T. Tanaka, M. Kozako, I.E.E.E. Trans, Dielectr. Electr. Insul. 12, 669 (2005)
M. Li, Z. Wei, L. Jiang, J. Mater. Chem. 18, 2276 (2008)
J. Duchet, R. Legras, S.D. Champagne, Synth. Met. 98, 113 (1998)
Y. Hou, L. Zhang, L.Y. Chen, P. Liu, A. Hirata, M.W. Chen, Phys. Chem. Chem. Phys. 16, 3523 (2014)
F. Gao, Z. Tang, Electrochim. Acta 53, 5071 (2008)
A.Y. Shenouda, H.K. Liu, J. Power Sources 185, 1386 (2008)
J. Song, M.Z. Bazant, J. Electrochem. Soc. 160, A15 (2013)
Y. Xia, W.K. Zhang, H. Huang, Y.P. Gan, C.G. Li, X.Y. Tao, Mater. Sci. Eng. B 176, 633 (2011)
C. Chen, G.B. Liu, Y. Wang, J.L. Li, H. Liu, Electrochim. Acta 113, 464 (2013)
H. Shu, X. Wang, Q. Wu, L. Liu, Q. Liang, S. Yang, B. Ju, X. Yang, X. Zhang, Y. Wang, Q. Wei, B. Hu, Y. Liao, H. Jiang, Electrochim. Acta 76, 120 (2012)
M. Jana, A. Sil, S. Ray, J. Phys. Chem. Solids 75, 60 (2014)
J. Lee, P. Kumar, J. Lee, B.M. Moudgil, R.K. Singh, J. Alloy Compd. 550, 536 (2013)
H. Shu, X. Wang, W. Wen, Q. Liang, X. Yang, Q. Wei, B. Hu, L. Liu, X. Liu, Y. Song, M. Zho, Y. Bai, L. Jiang, M. Chen, S. Yang, J. Tan, Y. Liao, H. Jiang, Electrochim. Acta 89, 479 (2013)
Y.H. Huang, K.S. Park, J.B. Goodenough, J. Electrochem. Soc. 153, A2282 (2006)
M. Winter, P. Novak, A. Monnier, J. Electrochem. Soc. 145, 428 (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sehrawat, R., Sil, A. Effect of solvents on electrochemical performance of polypyrrole coated LiFePO4/C cathode materials for Li-ion battery. J Mater Sci: Mater Electron 26, 5175–5185 (2015). https://doi.org/10.1007/s10854-015-3048-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3048-6