Abstract
Reduced titanium oxide Ti3O5 powder which was fabricated by a sol–gel process was added to lithium iron phosphate (LiFePO4) cathode electrodes for use in lithium-ion batteries and its performance was investigated. First discharging of the cathode electrode with Ti3O5 powder as the conductive additive keeps the capacity of 170.9 mAh g−1 at 0.1 C, 150.8 mAh g−1 at 0.5 C, 134.6 mAh g−1 at 1 C, and 107 mAh g−1 at 2 C, respectively, which is higher than that of the cathode electrode with acetylene black as the conductive additive, who keeps the capacity of 162 mAh g−1 at 0.1 C, 142.8 mAh g−1 at 0.5 C, 126.9 mAh g−1 at 1 C, and 105.8 mAh g−1 at 2 C, respectively. Over 100 cycles at 0.5 C, the LiFePO4 cathode electrode with Ti3O5 powder can maintain 77.5 % of its initial capacity, and the electrode with acetylene black shows 73.6 % capacity retention. The reason why the electrode with Ti3O5 additive shows better rate capability is that the Ti3O5 powder exhibits a relatively good electrical conductivity and shows a more homogeneous dispersion than acetylene black among the LiFePO4 particles during the cycles, in the investigation, a layer of suspected titanium oxide yarn-like thin film is discovered coating on the LiFePO4 particles of the cathode electrode with Ti3O5 powder after 100 cycles at 0.5 C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Olivine lithium iron phosphate (LiFePO4) with various advantages, such as high voltages (3.4 V vs. Li/Li+), relatively high theoretical capacity (170 mAh g−1), high thermal stability at high temperature, high safety, low cost and so on, has been considered as a promising candidate for a cathode material of lithium-ion batteries since it was first reported by Padhi et al. [1]. Unfortunately, the poor electronic conductivity and the low tap density have been main obstacles for the practical applications of LiFePO4.
At present, progressive efforts have been made by several research groups to overcome the low electrical conductivity of LiFePO4, including carbon coating [2, 3] and particle size minimization [4]. However, while researchers are focusing on the improvement of low electrical conductivity of LiFePO4, the low tap density of LiFePO4 has usually been overlooked and evaded. To effectively utilize the active material, the ratio of acetylene black often reaches 10 wt% in the blending of the cathode material with the binding polymer and electrically conductive additives. However, due to the low density of carbon, the required amount of acetylene black leads to considerable increase in volume resulting in a great decrease in the tap density and the following lower volumetric energy density of the LiFePO4 cathode electrodes in lithium-ion batteries. One way to improve the volume specific energy is to increase the cathode density by applying more compact and dense conductive additives, because this cell component has the largest volume and the heaviest weight. Therefore, there is a need for alternative conductive additives that may improve the performance and the tap density of the LiFePO4 cathode electrodes in lithium-ion cells.
Reduced titanium oxides materials are a series of substoichiometric oxides of titanium which are identifiable compounds and not simply doped titanium dioxide or casual mixtures of TiOx, where x is less than 2 [5]. They can be synthesized by heat-treating titanium dioxide at high temperature in a reducing atmosphere, usually in the presence of hydrogen [6] or carbon [7]. These oxides materials possess several advantages, including narrow band gap enabling absorption of visible light [8, 9], chemical stability, and high electrical conductivity comparable to that of graphite thanks to the mixed TiIII/TiIV valences [10]. Due to these favorable properties, a lot of academic and industrial laboratories have devoted considerable efforts to applications of these titanium oxides in various cells, for example, reduced titanium oxide Ti4O7 as a conductive additive for Zn electrodes in secondary Ni/Zn batteries [11], carbon-coated Ti9O17 nanobelts as anodes for Li-ion batteries and hybrid electrochemical cells [12] and reduced titanium oxides materials as a positive electrode conductive additive in Lead-acid batteries [5]. However, to our knowledge, this type of reduced titanium oxides materials as a conductive additive for Li-ion cells has not yet tested on LiFePO4 cathode electrodes in lithium-ion batteries.
Among the family of these titanium oxides materials, Ti3O5 has been considered to be well worth investigating and one of the most concerns for its excellent performance in electrical conductivity which can reach as high as 630 S cm−1 [13]. Herein, we adopt reduced titanium oxide Ti3O5 powder to replace acetylene black as the conductive additive for LiFePO4 cathode electrodes in lithium-ion cells.
2 Experimental
The LiFePO4 powder was purchased from Unanoergy Technology Co., Ltd, acetylene black was purchased from Shenzhen Poxon Machinery Technology Co., Ltd and Ti3O5 powder in this study was fabricated by a sol–gel process using tetrabutyl titanate as a precursor, dehydrated alcohol as an impregnant, and polyethylene glycol (PEG-600) as organic carbon source, and then by calcination at 1,000 °C for 4 h under high pure (99.999 %) argon atmosphere in an evacuated silica tube, as described in previous report [14]. The LiFePO4 cathode electrode contains 80 wt% dry basis LiFePO4 powder, 10 wt% conductive additive Ti3O5 or acetylene black, and 10 wt% poly(vinylidene fluoride) (PVDF) using N-methyl-2-pyrrolidone (NMP) as a solvent. The mixture slurry was coated on an aluminum foil and dried at 80 °C for 12 h. The charge and discharge characteristics were examined using a coin cell (CR2025 type), which consisted of a LiFePO4 cathode electrode, an electrolyte of 1 M LiPF6 in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DC) with a volume ratio of 1:2, a lithium metal negative electrode, and a Celgard 2500 separator. The CR2025 coin cells were assembled in an argon-filled glove box.
The crystalline structure of the samples was identified by a Rigaku Dmax-2000 XRD diffractionmeter with Cu Kα1 (λ = 0.154056 nm) radiation. The surface morphology of the samples was recorded using a Hitachi S-4800 field emission scanning electron microscope (FESEM). TG measurement of LiFePO4 powder was carried out by using a TG 209 F1 (NETZSCH Instrument). The size distribution of LiFePO4 powder was made at a temperature of 25 °C on a ZS90 nano particle and zeta analyzer (Malvern, UK). Brunauer–Emmett–Teller (BET) surface area of Ti3O5 powder and acetylene black powder was determined by a NOVA 4200e nitrogen adsorption instrument (Quantachrome Instruments, USA). The tap density of the samples was measured by a JZ-1 powder tap density tester (Shenzhen Sannuo Instrument Co. Ltd, China). The resistivity of Ti3O5 powder and acetylene black powder was measured by a FZ-2010 semiconductor powder resistivity meter (Shanghai Yi Yu Instrument Co., Ltd, China). Charge–discharge performances of the cells were evaluated by a LAND-CT2001A battery test system (Jinnuo Wuhan Corp., China) with voltage range from 2 to 4.2 V at room temperature.
To investigate the active material LiFePO4 after the completion of the cycles test, the cycled coin cells were dissembled in a glove box under Ar atmosphere, and the electrodes were rinsed with dehydrated alcohol and dried and stored in the glove box before analysis. Surface and particle morphology were examined by a Hitachi S-4800 FESEM, energy dispersive X-ray spectroscopy was used to analyze the surface compositions of electrodes.
3 Results and discussion
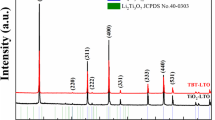
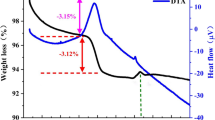
Figure 1a presents the XRD patterns of the cathode active material employed in this experiment and the standard PDF card of pure LiFePO4. The characteristic peaks of the employed cathode active material agree very well with that of pure LiFePO4 and no evidence of impurity phases could be observed, which denotes that the sample is olivine LiFePO4. The FESEM image of the employed LiFePO4 sample is shown in Fig. 1b. As can be seen, the LiFePO4 particles have a rice-like shape and there is a layer of carbon coating on the surface of LiFePO4 particles, and the average carbon-coated LiFePO4 particle size is 567.9 nm, as displayed in Fig. 1c. The carbon content of the employed carbon-coated LiFePO4 sample can be approximately estimated about 3.6 wt% through the TG curve in Fig. 1d.
The XRD pattern of the reduced titanium oxide powder sample is shown in Fig. 2a, almost all the peaks can be indexed as monoclinic Ti3O5 phase (PDF No.40-0806) except a weak peak of rutile type TiO2 is observed, illustrating that the reduced titanium oxide powder is relatively pure Ti3O5. Figure 2b, c shows the FESEM image of Ti3O5 powder and acetylene black powder, respectively. Acetylene black powder is more uniform in shape and size in comparison with that of Ti3O5 powder. However, the BET test appears a different result, as presented in Fig. 2d. The BET specific surface area of Ti3O5 particles is 145.5 m2 g−1, which is much larger than that of acetylene black powder who only exhibits 71.2 m2 g−1. This probably suggests that the Ti3O5 aggregates in Fig. 2b have a fluffy, porous structure and consist of smaller particles.
The composite electrode with 10 wt% Ti3O5 powder shows good rate capability. As shown in Fig. 3a, first discharging keeps the capacity of 170.9 mAh g−1 at 0.1 C, 150.8 mAh g−1 at 0.5 C, 134.6 mAh g−1 at 1 C, and 107 mAh g−1 at 2 C, respectively, which is better than that of the composite electrode with 10 wt% acetylene black, who keeps the capacity of 162 mAh g−1 at 0.1 C, 142.8 mAh g−1 at 0.5 C, 126.9 mAh g−1 at 1 C, and 105.8 mAh g−1 at 2 C, respectively. Over 100 cycles at 0.5 C, the LiFePO4 cathode electrode with Ti3O5 powder can maintain 77.5 % of its initial capacity, and the electrode with acetylene black shows 73.6 % capacity retention, as presented in Fig. 3b. The results demonstrate that Ti3O5 powder as the conductive additive is superior to acetylene black for LiFePO4 cathode electrodes in Li-ion cells. Moreover, Ti3O5 powder possesses a tap density of 1.46 g cm−3, which is more than eight times as high as that of acetylene black who only exhibits 0.17 g cm−3. Therefore, Ti3O5 additive can sufficiently increase tap density and volumetric energy density of the LiFePO4 electrode.
Figure 4 compares the FESEM images of the surface and morphology of electrodes with different conductive additives pre and post 100 cycles at 0.5 C. As shown in Fig. 4a, b, the surface and morphology of the electrode with acetylene black as the conductive additive remain unchanged during the cycles test, while, those of the electrode with Ti3O5 additive have been greatly changed after 100 cycles at 0.5 C. The surface of the cathode LiFePO4 substrate with Ti3O5 additive is found to be covered with a uniform thin layer of yarn-like material after the cycles test (Fig. 4d). The inset EDS results of Fig. 4d show the presence of Ti element in the selected rectangle region, which means there maybe exist titanium oxide film on the LiFePO4 particles, but its special chemical formula of titanium oxide is not sure just by the EDS results.
FESEM images of the LiFePO4 electrodes with different conductive additives pre and post 100 cycles at 0.5 C: a the electrode with acetylene black before the cycles test, b the electrode with acetylene black after the cycles test, c the electrode with Ti3O5 before the cycles test, d the electrode with Ti3O5 after the cycles test, and the inset is the corresponding EDS result of the rectangle selection region
Based on above analysis, the reasons for Ti3O5 additive improving the rate capability of LiFePO4 cathode electrodes in Li-ion cells can be attributed to that, firstly, Ti3O5 powder sample exhibits a relatively good electrical conductivity, its electrical resistivity is 4.7 × 10−3 Ω m, as a contrast, that of acetylene black is 5.6 × 10−4 Ω m. Despite the electric conductivity of Ti3O5 powder is lower than that of acetylene black, however, even more important is that Ti3O5 powder displays a more homogeneous dispersion among the LiFePO4 particles during the cycles than that of acetylene black powder, there forms a thin suspected titanium oxide film coating on the LiFePO4 particles after 100 cycles at 0.5 C, while the surface and morphology of the electrode with acetylene black has remained almost unchanged during the cycles test.
4 Conclusions
The analysis results indicate that Ti3O5 powder with a fluffy, porous structure exhibits a much larger BET specific surface area than acetylene black powder. The cathode electrode with Ti3O5 shows higher rate capability and cycling stability in comparison with the electrode with acetylene black. First discharging of the cathode electrode with Ti3O5 powder keeps the capacity of 170.9 mAh g−1 at 0.1 C, 150.8 mAh g−1 at 0.5 C, 134.6 mAh g−1 at 1 C, and 107 mAh g−1 at 2 C, respectively, and that of the cathode electrode with acetylene black is 162 mAh g−1 at 0.1 C, 142.8 mAh g−1 at 0.5 C, 126.9 mAh g−1 at 1 C, and 105.8 mAh g−1 at 2 C, respectively. Over 100 cycles at 0.5 C, the LiFePO4 cathode electrode with Ti3O5 powder as the conductive additive can maintain 77.5 % of its initial capacity, and the electrode with acetylene black shows 73.6 % capacity retention. The reasons can be summarized that, Ti3O5 powder exhibits a relatively good electrical conductivity and shows a homogeneous dispersion among the LiFePO4 particles during the cycles test, in the investigations, there exists a layer of suspected titanium oxide yarn-like thin film coating on the LiFePO4 particles after 100 cycles at 0.5 C.
In addition, Ti3O5 powder possesses a relatively high tap density of 1.46 g cm−3, much higher than that of acetylene black (0.17 g cm−3). Considering this and its relatively better rate capability, Ti3O5 powder has the potential to be applied to LiFePO4 cathode electrodes in lithium-ion batteries as the conductive additive.
References
A.K. Padhi, K.S. Nanjundaswamy, J.B. Goodenough, J. Electrochem. Soc. 144, 1188 (1997)
S.W. Oh, S.T. Myung, S.M. Oh, K.H. Oh, K. Amine, B. Scrosati, Y.K. Sun, Adv. Mater. 22, 4842 (2010)
C. Zhu, Y. Yu, L. Gu, K. Weichert, J. Maier, Angew. Chem. Int. Ed. 50, 6278 (2011)
S.-Y. Chung, Y.-M. Kim, S.-Y. Choi, Adv. Funct. Mater. 20, 4219 (2010)
J.R. Smith, F.C. Walsh, R.L. Clarke, J. Appl. Electrochem. 28, 1021 (1998)
X. Li, A.L. Zhu, W. Qu, H. Wang, R. Hui, L. Zhang, J. Zhang, Electrochim. Acta 55, 5891 (2010)
D. Regonini, A.C.E. Dent, C.R. Bowen, S.R. Pennock, J. Taylor, Mater. Lett. 65, 3590 (2011)
H.M. Liu, W.S. Yang, Y. Ma, J.N. Yao, Appl. Catal. A 299, 218 (2006)
W.Q. Han, Y. Zhang, Appl. Phys. Lett. 92, 203117 (2008)
Edmund G. Seebauer, Meredith C. Kratzer, Mater. Sci. Eng. R 55, 57 (2006)
Z. Luo, S. Sang, Q. Wu, S. Liu, ESC Electrochem. Lett. 2, A21 (2013)
W.Q. Han, X.L. Wang, Appl. Phys. Lett. 97, 243104 (2010)
A.A. Gusev, E.G. Avvakumov, AZh Medvedev, A.I. Masliy, Sci. Sinter. 39, 51 (2007)
Yun Chen, Jian Mao, J. Mater. Sci. Mater. Electron. 25, 1284 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Mao, J. Reduced titanium oxide Ti3O5 powder as a promising conductive additive for LiFePO4-based lithium-ion batteries. J Mater Sci: Mater Electron 25, 5153–5157 (2014). https://doi.org/10.1007/s10854-014-2285-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2285-4