Abstract
We synthesized some Eu(III) complexes {[Eu(dpa)(α-pc)(CH3OH)]·2CH3OH} (1), {[Eu(dpa)(2-ap)(CH3OH)]·2CH3OH}(2) and {[Eu(dpa)(2-hp)(CH3OH)]·2CH3OH}(3) [dpa = dipicolinic acid; α-pc = α-picolinic acid; 2-ap = 2-aminopyridine; 2-hp = 2-hydroxypyridine]. The structural characterization of the complexes were studied by elemental analysis, FTIR spectroscopy, SEM and powder X-ray diffraction studies (XRD) where the elemental analysis and FTIR results indicate the coordination of ligands with the Eu(III) ion. The X-ray diffraction patterns show the crystalline nature of complex (1) and amorphous nature of complexes (2) and (3) and the SEM micrographs also depict different morphologies of the complexes. The thermal properties of the synthesized complexes were studied by TG–DTA technique which indicates good thermal stability of the synthesized complexes. The optical properties were studied using Ultraviolet visible spectroscopy (UV–Vis) and Photoluminescence studies (PL) where photoluminescence measurements indicate that all the three complexes exhibit the characteristic emission bands of Eu(III) ion corresponding to 5Do → 7FJ (J = 0–4) transitions and it has also been observed that the intensity of emission is influenced by the effect of different secondary ligands. The most intense transition and the long radiative lifetime, quantum efficiency of the 5Do excited level of Eu(III) ion observed for the complex (2) with 2-ap as secondary ligand reflects the good sensitizing ability of ligand 2-ap. The optical properties of the obtained complexes can be well utilized for preparation of rare earth luminescent materials and fluorescence probes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The lanthanide complexes attract intense attention due to their superior optical properties and wide variety of potential applications i.e. biomedical assays and imaging, luminescence devices, ionic conductors and sensors [1–3]. The lanthanide based compounds are widely used as luminescent materials due to their extremely narrow emission bands with relatively long excited state lifetimes resulting from intraconfigurational 4f–4f transitions [4–6]. Due to the low molar extinction coefficient of the laporte forbidden f–f transition, the direct photoexcitation of the lanthanide ions becomes difficult [7, 8]. This can be overcome by the introduction of antenna which provides an alternative pathway for energy transfer and enriches the lanthanide emitting level [9–11]. These ligands transfer energy from the excited triplet state of ligand to the lowest emitting level of the lanthanide ion. The lanthanide ion then relaxes to ground state by the emission of energy [12–18]. Among the organic ligands, the pyridine derivatives are important because these can form diversity of coordinating structures including coordination polymers. The pyridine carboxylates act as good candidates due to existence of N and O mixed donor atoms which can coordinate to the metal ion in a bidentate chelating, bidentate bridging and unidentate manner [19, 20]. 2,6-pyridinedicarboxylic acid and α-picolinic acid have been found as promising ligands due to the stability of their Ln(III) complexes, strong fluorescence intensity with long excitation lifetimes [21]. The ligand 2,6-pyridinedicarboxylic acid is water soluble, commercially available having nitrogen and oxygen atoms to coordinate with the metal ion. Aminopyridine ligands also act as useful chelating agents for inorganic and organometallic applications and their derivatives can coordinate to the metal ions in a monodentate fashion through the N atom of the ring, however there are several works reported where the amino group also participates in coordination to the metal ion [22–24]. The hydroxypyridine ligands can also coordinate effectively to the metal ion through the N and O donor atoms and are expected to show good luminescent properties.
This article reports the synthesis of three europium complexes with dipicolinic acid (dpa) as a primary ligand and α-picolinic acid (α-pc), 2-aminopyridine (2-ap) and 2-hydroxypyridine (2-hp) as secondary ligands. Their structural, thermal and optical properties have also been studied.
2 Experimental
2.1 Materials and measurements
EuCl3(anh), dipicolinic acid (dpa, 99 %), α-picolinic acid (α-pc, 99 %), 2-aminopyridine (2-ap, 99 %), 2-hydroxypyridine (2-hp, 97 %) were procured from Sigma Aldrich and used as received without any further purifications. Methanol (CH3OH, 99.5 %) of analytical grade was used as such without any further purification. The elemental analysis was performed using GmbH Vario EL CHNS Elemental Analyzer. Fourier Transform Infra Red (FTIR) spectra were acquired on a Perkin Elmer Spectrometer in the range of 4,000–400 cm−1. Ultraviolet absorption spectra were recorded by Shimadzu UV-1601 spectrometer. Photoluminescence measurements were done on a Perkin Elmer LS55 fluorescence spectrophotometer equipped with a 150 W Xenon lamp as the excitation source and the photoluminescence decay measurements were performed on Horiba Jobin–Yvon FL-3-22 spectrofluorometer. Thermogravimetry (TG–DTA) data was obtained on Perkin Elmer diamond thermogravimetric analyzer at the heating rate of 10 °C/min under nitrogen atmosphere in the crucibles of Al2O3. The X-ray diffraction (XRD) measurements of the powdered samples were carried out by Bruker D8 diffractometer (40 mA–40 kV) using monochromatic Cu-Kα radiation (λ = 1.54 Å) over the 2θ range of 5–80°. The morphology of the synthesized complexes was studied by Scanning Electron Microscopy (SEM) using Carl Zeiss Scanning Electron Microscope.
2.2 Synthesis of europium complexes
The complex (1) was synthesized by taking dpa (1 mmol, 0.1679 g) and α-pc (1 mmol, 0.1231 g) in anhydrous methanol solution (10 ml) and the reaction mixture was stirred for half an hour. The pH of the solution was adjusted between 6 and 7 with dilute NaOH solution (1 mol L−1). EuCl3 (1 mmol, 0.2582 g) solution was added slowly dropwise to the ligand solution under stirring and the resulting solution was refluxed for 3–4 h at 70 °C. The white coloured precipitates were filtered, washed with methanol and then dried in a vacuum oven. The complex (2) was synthesized using EuCl3 (1 mmol, 0.2582 g), dpa (1 mmol, 0.1679 g) and 2-ap (1 mmol, 0.094 g) and complex (3) by taking EuCl3 (1 mmol, 0.2582 g), dpa (1 mmol, 0.1231 g) and 2-hp (1 mmol, 0.0951 g) following the same procedure as mentioned above.
3 Results and discussion
3.1 Elemental analysis results and solubility of complexes
The contents of C, H, N in the complexes were determined by elemental analysis and the content of Eu was determined by the volumetric titration with EDTA. The results are listed in Table 1. which show a good agreement between the experimental and calculated values. The complexes were found to be soluble in ethanol and water.
3.2 Infrared spectroscopy (FT-IR)
The infrared absorption spectra of the ligands and the complexes are shown in Fig. 1a–d and Fig. 2e–g. The FTIR spectra of the ligands dpa, α-pc and complex (1) showed that the absence of υ(C = O) stretching vibration at 1,700 cm−1 in dpa and at 1,605 cm−1 in α-pc suggested the coordination of COO− group of the ligand with rare earth ion. The mode of coordination of COO− group can be attributed to the difference between asymmetric and symmetric vibrations respectively. This difference was found to be 228 cm−1 in the complex which was larger than the ligand α-pc (152 cm−1) and smaller than that of the ligand dpa (242 cm−1) which revealed that the carboxylate group in the ligand dpa is coordinated in a bidentate chelating mode and the ligand α-pc in a unidentate manner. The complex (1) also showed a wide and strong band at 3,392 cm−1 due to υ(O–H) stretching vibration. For complex (2), the υ(C = O) stretching vibration of COO− group of ligand dpa disappeared and it shifted downward to 1,602 cm−1 in the complex which suggested the coordination of carboxylate group of ligand dpa to the rare earth ion through oxygen atom. The υ(N–H) stretching vibration peak at 3,397 cm−1 in ligand 2-ap also migrated to 3,290 cm−1 in the complex which revealed the coordination of N atom of NH2 group of ligand 2-ap to the rare earth ion. Also, the υ(C = N) stretching vibration at 1,487 cm−1 in ligand 2-ap shifted to 1,438 cm−1 in complex (2) indicating the coordination of N atom to the rare earth ion. In complex (3), the υ(C = O) stretching vibration at 1,700 cm−1 in dpa disappeared and a new band appeared at 1,606 cm−1, the υ(O–H) stretching vibration at 2,981 cm−1 in ligand 2-hp also shifted downward in the complex and appeared as a wide band at 2,924 cm−1.These downward shifts indicated the coordination of N and O atom of the ligand 2-hp to the rare earth ion. In all the three complexes, the Eu ← N bond stretching frequency appeared at 553, 593 and 579 cm−1 and the Eu ← O bond appeared at 424, 424 and 433 cm−1 for complexes (1), (2) and (3) respectively. These vibrations confirmed the formation of complexes as these could not be observed in the ligand FTIR spectra.
3.3 Thermogravimetric analysis (TG–DTA)
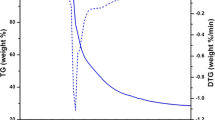
The TG–DTA curves of the complexes with heating rate of 10 °C/min in N2 atmosphere using α-Al2O3 as reference material are presented in Fig. 3a–c. As shown in Fig. 3a for complex (1), the mass loss observed in the first step of decomposition corresponded to the loss of solvent molecules and showed an endothermic peak at 89 °C. The second degradation occurred due to the loss of coordinated dpa and α-pc ligands and showed an endothermic peak at 469 °C. The thermal degradation continued and finally the complex decomposed through an endothermic effect at 578 °C. In the complex (2) as shown in Fig. 3b, the solvent loss appeared as an endothermic peak at 61 °C accompanied by the loss of coordinated 2-ap ligand through an endothermic effect at 148 °C. The complex finally decomposed through an endothermic peak at 486 °C. Finally, the complex (3) showed three endothermic peaks at 79, 196 and 481 °C due to the solvent loss, release of coordinated 2-hp ligand and final decomposition of the complex respectively.
3.4 UV–Vis absorption studies
The UV–Vis absorption spectra of the ligands and the complexes are recorded in Fig. 4a–c. The ligands dpa and α-pc showed absorption bands at 265 and 269 nm respectively, which could be assigned to π → π* transitions of the conjugated system of aromatic ring as shown in the Fig. 4a. In the complex (1), the absorption band observed at 267 nm was due to the overlap of absorption bands of ligands dpa and α-pc respectively and the intensity of this band was higher than that of dpa and lower than α-pc ligand. These observations suggested the formation of new compound by the coordination of ligands dpa and α-pc to the Eu(III) ion. As shown in the Fig. 4b, the ligand 2-ap exhibited mainly two absorption bands at 227 and 259 nm due to π → π* transitions of conjugated aromatic ring. The absorption bands at 227 and 267 nm in the complex (2) showed the red shift due to the expanded π—conjugated system resulting from the coordination of the ligands 2-ap and dpa to the Eu(III) ion. The band at 227 nm remains unchanged in the complex, however there was change in the intensity of absorption in the complex. In Fig. 4c, two absorption bands at 226 and 300 nm were observed for ligand 2-hp, which were attributed to π → π* transitions of the ligand and compared with the absorption bands of the ligands 2-hp and dpa, the complex (3) showed red shift in the absorption bands observed at 241 and 304 nm respectively. The intensity of absorption also got influenced after the coordination of Eu(III) ion with the ligands. These results suggested the coordination of Eu(III) ions with the ligands.
3.5 Photoluminescent studies
The room temperature emission spectrum of europium complexes as shown in Fig. 5 showed strong characteristic emission bands of Eu(III) ions in the visible region, which were attributed to the electronic transitions from the excited 5Do level to the ground 7FJ (J = 0–4) levels of Eu(III) ion. The emission bands at 580 nm (5D0 → 7Fo) and 650 nm (5Do → 7F3) were very weak since their corresponding transitions were forbidden both in magnetic and electric dipole fields. The luminescent intensity of (5D0 → 7F1) transition at 592 nm was relatively strong as this is magnetic transition which is independent of the coordinating environment of Eu(III) ion. The strongest emission observed at 615 nm (5Do → 7F2) is an induced electric dipole transition which is hypersensitive transition and is sensitive to the coordinating environment of Eu(III) ion. This transition is responsible for the brilliant red emission of europium complexes in the visible region. As shown in Fig. 5, the relative luminescent intensity of 5Do → 7F2 transition was strongest in complex (2) based on 2-ap as secondary ligand, however the weakest luminescent intensity was observed in complex (3). This could be explained by the non-radiative deactivation of energy of excited state as a result of their interaction with high frequency oscillators O–H group which act as efficient quencher of lanthanide ion luminescence in 2-hp ligand. These results showed that–NH2 group in 2-ap ligand can strongly sensitize the luminescence of Eu(III) ion as compared to the 2-pc and 2-hp ligands.
The luminescence decay curves of the complexes were obtained at 298 K by monitoring the 5D0 → 7F2 transition (615 nm) and are shown in Fig. 6. The decay profiles are monoexponential function indicating the presence of a single chemical environment around Eu(III) ion. The lifetime values were found to be 725, 825 and 675 μs for complexes (1), (2) and (3) respectively. The longest lifetime of complex (2) can be attributed to the efficient energy transfer and good sensitizing ability of 2-ap ligand while the shortest lifetime for complex (3) is related to the quenching effect of O–H oscillators in 2-hp ligand. According to the emission spectra and the lifetime of the Eu(III) excited state (5D0), the emission quantum efficiency (η) can be determined which can be calculated as follows:
where Ar and Anr are the radiative and non radiative transition rates respectively. Ar can be obtained by summing over the radiative rates A0J for 5D0 → 7FJ transitions (J = 0–4) of Eu(III) ion.
The experimental coefficients A0J can be calculated according to the following equation.
where A01 is Einstein coefficient of radiative emission between 5D0 and 7F1 energy levels of Eu(III) ion. Its value in vacuum can be taken as approximately 50 s−1 with an average index of refraction ‘n’ equals to 1.506 (A01 = n3 A01(vac)). I0J and I01 are the integrated intensities of 5D0 → 7FJ (J = 0–4) and 5D0 → 7F1 transitions of Eu(III) ion and ν0J, ν01 represents the energy barrier of the emission bands of Eu(III), 5D0 → 7FJ (J = 0–4) transitions [25, 26].The lifetime value (τ), radiative rate (Ar) and non radiative rate (Anr) are related by the following equation [27].
On the basis of above calculations, the quantum efficiencies (η) of the complexes are determined and are shown in Table 2. The results reveal that the complex (2) shows high value of lifetime (825 μs) and quantum efficiency (27.30 %) due to efficient energy transfer from the ligand 2-ap to Eu(III) ion whereas the complex (3) exhibits shortest lifetime owing to the possible quenching by −OH group present in the 2-hp ligand.
3.6 X-ray diffraction and morphological studies
The powder X-ray diffraction patterns of the complexes were recorded in a scanning range of 5–80° with CuKα radiation (λ = 1.5418 Å).The complex (1) displayed very sharp and few intense peaks indicating the crystalline nature of complex as shown in Fig. 7a. The diffractograms shown in Fig. 7b, c included weaker diffraction peaks indicating the amorphous nature of the complexes (2) and (3). Figure 8a–c shows the SEM images of the obtained complexes and depicts the distinguishable morphologies of the complexes. The complex (1) showed rod like morphology while the complex (2) displayed agglomerated like structure. The complex (3) indicated sponge like structure containing aggregated particles.
4 Conclusions
In the present paper, three europium complexes based on dpa as primary ligand and α-pc, 2-ap and 2-hp as secondary ligands have been synthesized and characterized using elemental analysis, FT-IR, TG–DTA, UV–Vis, PL, XRD and SEM. The elemental analysis and FTIR reveals the coordination of the ligands with the rare earth ion. The powder X-ray diffraction studies show the crystalline nature of complex (1) and amorphous nature of complex (2) and complex (3). The SEM micrographs display different morphologies of the obtained complexes. The complexes show the characteristic emission bands of Eu(III) ion in visible region at 580, 592, 615, 650 and 698 nm for 5D0 → 7FJ (J = 0–4) transitions respectively. The optical properties of the complexes are influenced by the secondary ligands as studied by the photoluminescence spectra and decay measurements, where the complex (2) with 2-ap as secondary ligand exhibits strongest emission intensity and relatively longer luminescence lifetime, quantum efficiency as compared to the complex (1) and complex (3).The UV–Vis absorption studies show the remarkable changes in the absorption wavelength of complexes, thus indicating the formation of complexes by the coordination of ligands with Eu(III) ion. All the results conclude that the luminescent property of the complexes can be well utilized for the preparation of organic light emitting diodes and fluorescence probes.
References
J.H. Xue, X.H. Hua, L.M. Yang, W.H. Li, Y.Z. Xu, G.Z. Zhao, G.H. Zhang, K.X. Liu, J.E. Chen, J.G. Wu, Synthesis, crystal structures and luminescence properties of europium and terbium picolinamide complexes. Chin. Chem. Lett. 25, 887–891 (2014)
L. Meijuan, W. Xiaoping, T. Qiang, L. Qidan, Luminescence properties of polymers containing europium complexes with 4-tert-butylbenzoic acid. J. Rare Earths 31, 950–956 (2013)
K.P. Zhuravlev, V.I. Tsaryuk, I.S. Pekareva, J. Sokolnicki, Z.S. Klemenkova, Europium and terbium ortho-, meta-, and para-methoxybenzoates: Structural peculiarities, luminescence, and energy transfer. J. Photochem. Photobiol. A 219(219), 139–147 (2011)
M. Rasanen, H. Takalo, J. Rosenberg, J. Makela, K. Haapakka, J. Kankare, Study on photophysical properties of Eu(III) complexes with aromatic β-diketones—Role of charge transfer states in the energy migration. J. Lumin. 146, 211–217 (2014)
M.G. Lahoud, L.F. Marques, P.B. Da Silva, C.A. De Jesus, C.C. Da Silva, J. Ellena, R.S. Freits, M.R. Davolos, C.G. Frem Regima, Synthesis, crystal structure and photoluminescence of a binuclear complex of europium(III) containing 3,5-dicarboxypyrazolate and succinate. Polyhedron 54(54), 1–7 (2013)
G. Zucchi, O. Maury, P. Thurey, M. Ephritikhine, Structural diversity in Neodimium bipyrimidine compounds with near infrared luminescence: From mono and binuclear complexes to metal-organic frameworks. Inorg. Chem. 47, 10398–10406 (2008)
L. Armelao, S. Quici, G.A. Barigelletti, G. Bottaro, M. Cavazzini, E. Tondello, Design of luminescent lanthanide complexes : From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 254, 487–505 (2010)
G.F. De Sa, O.L. Malta, C. de Mello Donega, A.M. Simas, R.L. Longo, P.A. Santacruz, E.F. Da Silva Jr., Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coord. Chem. Rev. 196, 165–195 (2000)
H.A. Azab, A. Duerko, Z.M. Anwar, B.H.M. Hussein, M.A. Rizk, Luminescence recognition of different organophosphorus pesticides by the luminescent Eu(III)-pyridine-2,6-dicarboxylic acid probe. Anal. Chim. Acta 759, 81–91 (2013)
H. Zheng, D. Gao, F. Zhenxing, E. Wang, Y. Lei, Y. Tuan, M. Cui, Fluorescence enhancement of Ln3+ doped nanoparticles. J. Lumin. 131, 423–428 (2011)
L. Zhang, Y. An, W. Ahmad, Y. Zhou, Z. Shi, X. Zheng, A new quarternary luminescence enhancement system of Eu-N-(3-methoxysalicylidene)-2-aminopyridine-1,10-phenanthroline-Zn and its application in determining trace amounts of Eu3+ and Zn2+. J. Photochem. Photobiol. A 252, 167–173 (2013)
Z. Hnatejko, G. Dutkiewicz, M. Kubicki, S. Lis, New complexes of cobalt(II) ions with pyridine carboxylic acid N-oxides and 4,4′-byp. J. Mol. Struct. 1034, 128–133 (2013)
G.X. Liu, Y.Y. Xu, X.M. Ren, S. Nishihara, R.Y. Huang, Self–assembly of 3d-4f coordination frameworks based on pyridine-3,5-dicarboxylic acid: Synthesis, crystal structuresand luminescence. Inorg. Chim. Acta 363, 3727–3732 (2010)
R. Tang, Q. Zhao, Z.E. Yan, Y.M. Luo, Synthesis of novel derivatives of pyridine-2,6-dicarboxylic acid. Synth. Commun. 36, 2027–2034 (2006)
Q. Yue, J. Yang, G.H. Li, G.D. Li, W. Xu, J.S. Chen, S.N. Wang, Three dimensional 3d-4f Heterometallic coordination polymers: Synthesis. Struct. Magn. Prop. Inorg. Chem. 44, 5241–5246 (2005)
J.C.G. Bunzli, Lanthanide Luminescence for biomedical analyses and imaging. Chem. Rev. 110, 2729–2755 (2010)
H. Tsukube, S. Shinoda, Lanthanide complexes in molecular recognition and chirality sensing of biological substrates. Chem. Rev. 102, 2389–2403 (2002)
D.T. De Lill, D.A. Bettencourt, C.L. Cahill, Exploring Lanthanide luminescence in Metal-Organic Frameworks: Synthesis, Structure and Guest-Sensitized Luminescence of a mixed Europium/Terbium-Adipate Framework and a Terbium-Adipate Framework. Inorg. Chem. 46, 3960–3965 (2007)
D. Ma, W. Wang, Y. Li, J. Li, C. Daiguebonne, G. Calvez, O. Guillou, In situ 2,5-pyrazinedicarboxylate and oxalate ligands synthesis leading to a microporous europium-organic framework capable of selective sensing of small molecules. Cryst. Eng. Comm. 12, 4372–4377 (2010)
J. Huang, Y. Xu, X. Chen, D. Xu, Y. Xu, Q. He, Synthesis, characterization and properties of some rare earth complexes with 2,6-pyridine dicarboxylic acid and α-Picolinic acid. J. Rare Earths 30, 586–591 (2012)
S. Mistri, E. Zangrando, S.C. Manna, Cu(II) complexes of pyridine-2,6-dicarboxylate and N-donor neutral ligands: Synthesis, crystal structure, thermal behavior, DFT calculation and effect of aromatic compounds on their fluorescence. Inorg. Chim. Acta 405, 331–338 (2013)
S.P. Jose, S. Mohan, Vibrational spectra and normal co-ordinate analysis of 2-aminopyridine and 2-aminopicoline. Spectrochimica. Acta. Part A. 64, 240–245 (2006)
D. Wang, Y. Pi, C. Zheng, L. Fan, Y. Hu, X. Wei, Preparation and photoluminescence of some europium (III) ternary complexes with β-diketone and nitrogen heterocyclic ligands. J. Alloy. Compd. 574, 54–58 (2013)
R. Lyszczek, L. Mazur, Polynuclear complexes constructed by lanthanides and pyridine-3,5-dicarboxylate ligand: Structures, thermal and luminescent properties. Polyhedron 41, 7–19 (2012)
M.E. Mesquita, S.S. Nobre, M. Fernandes, R.A.S. Ferreira, S.C.G. Santos, M.O. Rodrigues, L.D. Carlos, V. de Zea Bermudes, Highly luminescent di-ureasil hybrid doped with a Eu(III) complex including dipicolinate ligands. J. Photochem. Photobiol. A 205, 156–160 (2009)
M.R. George, C.A. Golden, M.C. Grossel, R.J. Curry, Modified dipicolinic acid ligands for sensitization of Europium(III) luminescence. Inorg. Chem. 45, 1739–1744 (2006)
A.A. Picot, P.L. Baldeck, C. Andraud, O. Maury, Design of dipicolinic acid ligands for the two-photon sensitized luminescence of europium complexes with optimized cross- sections. Inorg. Chem. 47, 10269–10279 (2008)
Acknowledgments
The authors would like to thank Guru Gobind Singh Indraprastha University, New Delhi for providing financial support as Indraprastha Research Fellowship for research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, G., Narula, A.K. Synthesis and optoelectronic properties of three Eu(III)-dipicolinate complexes based on α-picolinic acid, 2-aminopyridine and 2-hydroxypyridine as secondary ligands. J Mater Sci: Mater Electron 26, 1009–1017 (2015). https://doi.org/10.1007/s10854-014-2497-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2497-7