Abstract
Luminescent nano-pigments based on strontium aluminates doped with various amounts of europium rare earth ion (Sr4Al14O25:Eu2+) were synthesized by solution combustion method which involves the exothermic reaction of an oxidizer and an organic fuel. It is a suitable method for producing chemically homogenous and pure powders with very fine particle size. Phases studies by X-ray diffractometer indicated formation of pure phase Sr4Al14O25 with crystallite sizes about 52.46 nm and peak shift of about 2θ = 0.26° due to Eu2+ doping. scanning electron microscope micrographs also revealed that the particles have irregular morphology and wide particle size distribution. The emission spectrum showed main emission peak at about 480 nm. Energy transfer mechanism between the two emission centers and the effect of Eu2+ ion concentration on emission spectrum was studied and it was deduced that the maximum emission intensity occurred at 4 at% of Eu2+. Absorption spectrum of the samples were lower than 0.5 %, especially in emission region of the doped pigments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, white light emitting diodes (WLEDs) have attracted much attention, since they have less negative effect on environment and are more cost effective [1, 2]. Compared to conventional lighting devices, these diodes have some advantages, including low applied voltage, high power efficiency and brightness and longer life [3–5]. Two methods have been used to generate white light. One is by utilizing primary tricolor phosphors excited by near UV–LED chips and the other by using blue LED with yellow emitting phosphors. Nevertheless, these two methods have some disadvantages including high color tolerance and low color rendering index (CRI). To overcome these problems various phosphors doped with rare earth ions have been studied to formulate new phosphors for this purpose [2]. Among the studied phosphors, alkaline earth aluminates doped with rare earth elements have proven to be useful, as they have strong luminescence in blue- green to red regions of visible spectrum [2, 6, 7]. They have high quantum yield, and are safe and chemically stable [7].
In SrO–Al2O3 system, there are six known phosphor hosts, namely Sr4Al14O25 [1], SrAl2O4 [2], Sr2Al6O11 [4, 7, 8], SrAl12O19 [1, 4, 7, 8], Sr3Al2O6 [4, 8], and SrAl4O7 [8]. Recently, strontium aluminates, especially, SrAl2O4 and Sr4Al14O25 doped with rare earth ions (Eu2+, Ce3+) have been investigated as proper phosphors with high brightness and broad and intense charge transfer bands or host absorption bands in near UV regions. Sr4Al14O25 demonstrates strong host absorption band ranging from 260 to 340 nm [7]. Moreover, Eu2+ is a lanthanide element with f1 configuration [9] and is, therefore, capable of creating luminescence from red to UV-region owing to strong crystal field dependence of 5d–4f transition energy [8, 9]. Sr4Al14O25:Eu2+ also has been investigated for its outstanding properties, including high quantum efficiency and bright blue–green LEDs fabricated by combining NUV InGaN based LEDs with this phosphor [6].
Different processes such as solid state reaction [1–3, 5, 7], sol–gel [1], co-precipitation [10], microwave synthesis [1] and solution combustion [9] are among the methods used for synthesis of these phosphors. Compared to the samples obtained by conventional solid state reaction method, the phosphor materials synthesized by wet chemical method have some advantages such as low calcinations temperature and very fine particle size.
Crystallographic structure of Sr4Al14O25 were reported for the first time by Nadzhina et al. [11]. Sr4Al14O25 has orthorhombic structure with space group Pmma, and a = 24.785, b = 8.487 and c = 4.866 Å. The structure consists of layers made up of AlO6 octahedra separated by a double layer of AlO4 tetrahedra [6]. In Sr4Al14O25 structure, there are two different strontium sites: the Sr1 site inside an oxygen-polyhedron composed of six O atoms and the Sr2 site inside a complicated oxygen-polyhedron composed of eight O atoms [6, 11].
In this work, synthesis of Sr4Al14O25:Eu2+ phase by solution combustion method and studying its luminescent properties have been investigated.
2 Experimental
A series of samples were prepared by solution combustion route. All of the starting materials were of reagent grades. At first, stoichumetric amounts of Sr(NO3)2(99 %), Al(NO3)3.9H2O, (99 %), Eu(NO3)3, [prepared by adding HNO3 into proper amounts of Eu2O3 (99 %) until a transparent solution was obtained] were mixed with double distilled water on a magnetic stirrer. Urea was added as fuel with oxidizer to fuel ratio of about 2.5. By adding some ammonia, pH of the solution was fixed at 4. After that the solution was heated in hot plate at about 80 °C, until a gel was formed. The viscous gel was inserted directly inside a furnace which was preheated to 600 ± 2 °C. In this way, the gel was rapidly dehydrated and transformed into a foam. Then, it was self-ignited and a white fluffy mass was produced. After light crushing the product by a mortar and pestle, it was heat treated at 1,350 °C in a tube furnace under reductive atmosphere of active carbon and N2/Ar gas.
The powder diffraction data was collected by a XRD (Philips PW3040/60, Cu Kα:1.54056 Å, 40 kV and 30 mA, Holland), and the particle size and morphology was determined by a scanning electron microscope (SEM) (S4160, Hitachi, Japan).The samples were coated with gold by a DC sputtering device. The photoluminescence emission and excitation spectra were carried out with a Perkin-Elmer spectrophotometer LS50B equipped with Xenon lamp as excitation source. The absorption spectra were recorded on a UV–Visible spectrophotometer (UV1800, Ray Leigh, Japan). Crystallite sizes were calculated by Scherer equation:
where d is crystallite size (Å), λ is X-ray wavelength (1.54056 Å), β is full width at half maximum (FWHM) and θ is the angle [1].
3 Results and discussion
3.1 Phase analysis
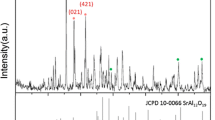
The X-ray diffractometer (XRD) patterns of the sample after combustion and heat treatment at 1,350 °C are shown in Fig. 1. The patterns confirm the partial and complete formation of Sr4Al14O25 (JCPDS 01-074-1810) before and after combustion, respectively. The weak intensity of the peaks before heat treatment is believed to be due to the short time of combustion process (about thirty-seconds). Fig. 1a also shows the presence of minor phases from the SrO–Al2O3 system, including SrAl2O4 (JCPDS 00-010-0061), SrAl12O19 (JCPDS 00-026-097), and other unrecognizable phases, which almost disappeared after heat treatment at 1,350 °C.
The crystallite sizes of the powders before and after heat treatment were about 30.25 and 52.46 nm, respectively as determined by using Scherer equation.
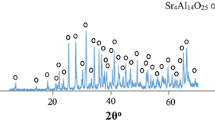
XRD patterns of the doped and undoped samples are shown in Fig. 2. The patterns revealed that Eu2+ ion doping had no effects on crystallization of the host material.
Since Eu2+ radius (0.112 nm) is smaller than Sr2+ radius (0.114 nm) [6], by doping Eu2+ activator ions into Sr4Al14O25 matrix, the host structure contracts and this contraction affects the lattice parameter and XRD pattern peak positions, and causes a peak shift to higher angles. This peak shift is shown in Fig. 3. The value of this peak shift caused by doping found to be about 2θ = 0.26° compared to undoped XRD pattern.
3.2 Spectroscopy
The luminescence spectrum of the 4 % Eu2+ doped sample is shown in Fig. 4. The spectrum shows a relatively broad excitation spectra with two maxima at about 250 and 350 nm. This broad excitation spectra could be related to electron transition from 4f7 ground state to the 4f65d1 excited state of Eu2+ [6].
When the excitation wavelength was 250 nm, the emission spectrum (Fig. 5) does not show very sharp emission spectra, but by excitation with 350 nm wavelength, the emission spectrum (Fig. 6) showed an intense green emission with a maximum at 480 nm, which could be related to the 4f 65d1 → 4f7 transition of Eu2+ ion [6, 11].
Figure 6 shows the effects of activator ion concentration on the emission spectra of Sr4Al14O25:Eu2+ pigment. It can be seen that, emission intensity first increases with increasing activator ion concentration up to 4 at% Eu2+. After which quenching effect in host structure occurs which leads to decrease of emission intensity.
As reported by many research groups [1, 2, 8, 10, 11], concentration quenching occurs when activator concentration exceeds a proper amount (usually about a few weight percent). Figure 7 shows variation of peak emission intensity with Eu2+ ion concentration. Previous research studies showed that in some phosphors activated with rare earth ions, the concentration quenching effect is very small and in some phosphors all of replaceable ions could be replaced with activator ions [12]. In Sr4Al14O25:Eu2+, it is predicted that with further addition of activator ions (Eu2+) the emission intensity does not show sensible variations (Fig. 7).
In the spectrum shown in Fig. 6, there is also another weak emission peak at about 400 nm. Existence of two emission band using only one activator ion could be related to the presence of two different crystallographic sites for Sr2+ ions in the Sr4Al14O25 host structure as reported by Wu and Zhang et al. [6, 11]. Since Eu2+ ion radius (0.112 nm) is nearly similar to Sr2+ (0.114 nm) [6] or (0.113 nm) [8], Eu2+ ions could be easily substituted for Sr2+ [6, 8], leading to two types of Eu2+ sites in the matrix structures [6, 8, 11].
The excited states of 5d in Eu2+ ion are influenced by crystal field effects [8], as these different excited states of Eu2+ lead to two different crystal fields around them, and therefore two different emission peaks in emission spectra.
It was reported that although the number of the both sites is equal, the 480 nm emission is more intense [6, 8]. This might be due to energy transferring from Eu2+ ions with emission at 400 nm to Eu2+ ions emitting at 480 nm [8]. Another reason for this phenomenon could be overlapping 400 nm emission peak with excitation spectra [6]. It can be seen from Fig. 7 that decrease of the emission intensity with increasing Eu2+ above about 4 % ion concentration is too high, but finally it levels off.
Of course, when the concentration quenching due to cross-relaxation (relaxation due to resonant energy transfer between the same element atoms or ions) occurs on a particular level among several emitting levels, the emission color of phosphor changes with activator concentration. The change in emission color which occurs by diminishing one of the emission wavelengths, is caused by cross relaxation between emission levels [12]. Hence, in relation to the Sr4Al14O25: Eu2+ pigment, the energy transfer from 400 nm emission center to 480 nm could be the reason for color changing with increasing Eu2+ concentration greater than 1 at%.
Emission spectra at different excitation wavelengths of 350, 390 and 400 nm are shown in Fig. 8.
As could be seen from Figs. 5, 8 with various excitation wavelengths, the main emission wavelength did not change. Excitation by shorter wavelengths (250 nm) led to the significant increase of the emission intensity, which is a result of high excitation intensity at this wavelength. But under higher excitation wavelengths (350, 390 and 400 nm) the emission intensity decreases to half of it at 390 nm excitation. This could be explained by lower excitation intensity at 390 nm in Fig. 4. But from another point of view, it can be seen that the produced pigment shows emission peak at different excitation wavelengths, but main emission peak always occurs at a constant wavelength of 480 nm. This shows that emissions caused by relaxation of excited electrons, have one exact level. But, in excitation with shorter wavelengths, excited electron after releasing part of its energy in form of heat recombines to proper level, causing emission. With the same reason, broadening of the emission spectra could be explained, that is emission caused by excited electrons recombination could cause other emission wavelengths in addition to main or maximum emission wavelength, so emission spectra become wider. The reason of emission at the same wavelength produced by longer wavelengths could be explained by the presence of different energy levels for holes inside pigment structure.
Figure 9 shows SEM of the Sr4Al14O25:4 at% Eu2+ powders before and after heat treatment at 1,350 °C. It can be seen that the pigment has a wide particle size distribution of 84 nm–2 μm before heat treatment and 152 nm–3.86 μm after heat treatment.
Energy efficiency is one of the most important factors in the field of luminescent materials application at photoelectrical instruments. Parameters affecting energy efficiency are given in the following equation:
where, r, is backscatter coefficient, hν is the mean photon energy of the photons emitted, βEg is the energy needed to generate a thermalized electron–hole pair (β being a pure number and Eg being the band gap energy), ηt is the transfer efficiency of electron–hole pairs to activators or sensitizers, ηact is the quantum efficiency of the activator ions, and finally ηesc is the ratio between photons leaving the material and photons generated in the material. Absorption of the emission wavelength by matrix has a reverse relation with (ηesc), that is, the less matrix phase absorption, the more phase ηesc and energy efficiency will be [13].
The necessary requirement for phosphors applications for NUV–LED solid state light is the high absorption rate in the 350–410 nm [6], hence, Sr4Al14O25: Eu2+ with wide excitation spectra in the 200–420 nm (Fig. 4), would be a suitable choice for application in LEDs which could be excited with violet photons. Photon absorption at emission peak (about 480 nm) determined by absorption spectra of Sr4Al14O25:4 at% Eu2+ pigment (Fig. 10). As observed, it shows less than 0.5 % photon absorption at emission peak of Sr4Al14O25:Eu2+ pigment. Therefore, the synthesized pigments have also high efficiency rate.
4 Conclusions
XRD phase analysis confirmed the formation of high purity Sr4Al14O25:Eu2+ phase with crystallite sizes of about 52.46 nm. It was observed that by doping Eu2+, due to the matrix phase structure contraction, peaks of the XRD peaks shifted to higher angles, but it did not affect phase formation. SEM analysis showed a relatively wide particle size distribution. The emission spectra of Sr4Al14O25: Eu2+ pigments showed two emission peaks, one with high intensity at 480 nm and the other one with lower intensity at 400 nm. Energy transfer between two emission centers and also overlapping the excitation spectra with emission peak at 400 nm were found to be major reasons for the intensity difference. The maximum emission intensity was found at Eu2+ ion concentration about 4 at%. It was concluded that Sr4Al14O25: Eu2+ pigment is a suitable choice for optoelectronic device applications such as NUV/VUV and LED/PDPs, because of its wide excitation range and high emission intensity and high luminescence efficiency rate due to low matrix phase electromagnetic absorption.
References
M. Elsagh, M. Rajabi, E. Amini, Characterization of SrAl2O4: Eu2+, Dy3+ phosphor nano-powders produced by microwave synthesis route. J. Mater. Sci. Mater. Electron. 25, 1612–1619 (2014)
K. Pavani, J. Suresh Kumar, T. Sasikala, B.C. Jamalaiahb, H.J. Seo, L. Rama Moorthy, Luminescent characteristics of Dy3+ doped strontium magnesium aluminates phosphor for white LEDs. Mater. Chem. Phys. 129, 292–295 (2011)
Y.D. Xu, D. Wang, L. Wang, N. Ding, M. Shi, J.G. Zhong, S. Qi, Preparation and luminescent properties of a new red phosphor (Sr4Al14O25:Mn4+) for white LEDs. J. Alloy. Compd. 550, 226–230 (2013)
S.H. Han, Y.J. Kim, Luminescent properties of Ce and Eu doped Sr4Al14O25 phosphors. Opt. Mater. 28, 626–630 (2006)
W. R Liu, C. C Lin, Y. C Chiu, Y. T Yeh, S. M Jang, R. S Liu, “Luminescence properties of green-emitting phosphors-Sr4Al14O25:Eu2+ for LED applications”, The 13th Asia Pacific confederation of chemical engineering congress, Taipei, (2010)
Z. Wu, J. Shi, J. Wang, M. Gong, Q. Su, Synthesis and luminescent properties of Sr4Al14O25:Eu2+ blue–green emitting phosphor for white light-emitting diodes (LEDs). J. Mater. Sci. Mater. Electron. 19, 339–342 (2008)
H.N. Luitel, T. Watari, R. Chand, T. Torikai, M. Yada, Photoluminescence properties of a novel orange red emitting Sr4Al14O25:Sm3+ phosphor and PL enhancement by Bi3+ co-doping. Opt. Mater. 34, 1375–1380 (2012)
L. Qun, Z. Junwu, S. Feilong, Energy transfer mechanism of Sr4Al14O25:Eu2+ phosphor. J. Rare Earths 28, 26–29 (2010)
S.K. Sharma, S.S. Pitale, M.M. Malik, R.N. Dubey, M.S. Qureshi, Luminescence studies on the blue–green emitting Sr4Al14O25:Ce3+ phosphor synthesized through solution combustion route. J. Lumin. 129, 140–147 (2009)
Z. Wu, M. Gong, J. Shi, Q. Su, Comparative investigation on synthesis and luminescence of Sr4Al14O25:Eu2+ applied in InGaN LEDs. J. Alloy. Compd. 458, 134–137 (2008)
S. Zhang, R. Pang, Ch. Li, Q. Su, Green photoluminescence, but blue afterglow of Tb3+ activated Sr4 Al14 O25. J. Lumin. 130, 2223–2225 (2010)
S. Shionoya, W.M. Yen, Phosphor handbook (CRC Press, London, 1999), pp. 108–110
C. Ronda, Luminescence: From theory to applications (WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2008)
Acknowledgments
The authors would like to thank INSF of Iran for complete financial support provided for this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zand, S.K., Baghshahi, S. & Rajabi, M. Synthesis of Sr4Al14O25:Eu2+ green emitting luminescent nano-pigment by solution combustion method. J Mater Sci: Mater Electron 25, 4412–4417 (2014). https://doi.org/10.1007/s10854-014-2181-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2181-y