Abstract
An efficient blue–green emitting phosphor, Sr4Al14O25:Eu2+, was prepared by solid-state reaction. X-ray powder diffraction (XRD) analysis confirmed the formation of Sr4Al14O25:Eu2+. Field-emission scanning electron-microscopy (FE-SEM) observation indicated that the microstructure of the phosphor consisted of irregular fine grains with an average size of about 8–10 μm. Photoluminescence measurements showed a broad absorption band between 300 and 450 nm which was efficiently excited by near-ultraviolet (NUV) LEDs (350–410 nm) and a strong emission band peaking at 491 nm. A bright blue–green LED with chromatic coordination (0.176, 0.412) was fabricated by incorporating the phosphor with an InGaN-based NUV chip, which indicates that Sr4Al14O25:Eu2+ is a good candidate phosphor for application in white LEDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since Nakamura et al. [1] fabricated a blue-emitting GaN LED in 1993 and the first commercial white LED solid-state lighting was developed using this blue-emitting LED in 1997 [2], more and more interest has been focused on GaN-based white LEDs. There have been some detailed studies on the integration of the blue InGaN LED and the yellow YAG phosphor, as this creates white light [2–4]. Excitingly, the efficiency of white LED lighting has already exceeded that of the incandescent lamps and now is competitive with fluorescent lamps [5–7]. However, this type of white light has poor color rendering index caused by the color deficiency in the red and blue–green phosphor. Furthermore, it is hard to improve power of blue-emitting GaN chip and find other suitable phosphors excited at 460 nm efficiently, besides the YAG:Ce3+ phosphor and some sulfide phosphors [8–12]. To overcome these problems, NUV LED-RGB white LEDs have been suggested for general illumination because of the remarkable progress in the development of white LEDs using InGaN chip whose emission bands shift to NUV range around 400 nm [13–16]. The necessary requirement for phosphors that are used for NUV-LED solid-state light is the high absorption in the 350–410 nm. Conventional phosphors used in fluorescent lighting are not ideal for solid-state light because they have poor absorption in the NUV to blue region. So it is necessary to identify suitable phosphors in the RGB regimes.
Strontium aluminates, doped with Eu2+, have been studied for a long time for their excellent properties such as high quantum efficiency [17] long persistence of phosphorescence [18] and good stableness [19]. In particular, SrAl2O4:Eu2+ phosphor has been studied in depth and applied intensively [20]. Recently, a bright green LED device fabricated by incorporating the SrAl2O4:Eu2+ phosphor with an InGaN-based NUV chip was reported by our research group [21]. As shown in this paper, SrAl2O4:Eu2+ is an excellent phosphor applied in white LEDs. Besides the well-known strontium monoaluminate SrAl2O4, it has been reported that Sr4Al14O25:Eu2+ phosphors as a green and blue emitter have even higher quantum efficiency [17]. However, little attention was paid to its application on white LED devices. In this article, the crystallization, morphologies, particle size and luminescent properties of Sr4Al14O25:Eu2+ prepared by solid-state method were investigated, and a bright blue–green LED was fabricated by the combination of NUV InGaN based LED with this phosphor.
2 Experimental
A series of samples, 4Sr1-x Eu x O·7Al2O3 (x = 0.005, 0.010, 0.015, 0.020, 0.025, 0.030, 0.035), were prepared by conventional solid-state reaction technique. Firstly, the starting materials which include SrCO3 (AR), Al(OH)3 (AR), Eu2O3 (99.99%) were taken in an agate mortar in stoichiometric molar ratio and a little H3BO3 (AR) was added as flux. After a fully grinding, the mixtures were put into corundum crucibles respectively and calcined in an electric furnace at 1350°C for 3 h in a weak reductive atmosphere of active carbon. Finally, the samples were gained by a fully grinding in an agate mortar after cooling to room temperature naturally.
Crystal phase identification was carried out on an X-ray diffractometer (D/max – IIIA, RIGAKU Corporation of Japan) using 40 kV, 20 mA, and Cu-K α radiation (1.5406 Å). Morphology and size of the calcined particles were observed by Field-emission scanning electron microscopy (FE-SEM, JSM-6330F, JEOL Corporation of Japan). Platinum power was sprayed onto the sample surface before FE-SEM observation. Excitation and emission spectra of the phosphors were measured on a Fluorolog-3-21 spectrometer (JOBIN YVON, America) at room temperature and a 450 W xenon lamp was used as the excitation source. Spectra and CIE color coordinates of the LEDs were recorded on the LED-1100 Spectral/Goniometric Analyzer from Labsphere Inc.
3 Result and Discussion
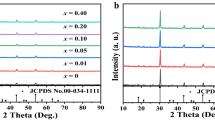
The crystal structure of Sr4Al14O25 has been refined to be orthorhombic, space group Pmma, with a = 24.785 Å, b = 8.487 Å and c = 4.866 Å [22] and the results were also been confirmed by following studies [23–25]. The crystal structure of Sr4Al14O25 was shown in Fig. 1 [26]. From Fig. 1, it is easy to find that the orthorhombic Sr4Al14O25 consists of layers made up of AlO6-octahedra separated by a double layer of AlO4-tetrahedra and there are two different strontium sites in Sr4Al14O25: the Sr1 site lies in the oxygen-polyhedron composed of six O atoms and the Sr2 site lies in the complicated oxygen-polyhedron composed of eight O atoms. The XRD patterns of our samples, 4Sr1-x Eu x O·7Al2O3 (x = 0.005 – 0.035), are shown in Fig. 2, which indicated that most of peaks can be indexed to the phases of Sr4Al14O25 (PDF 52-1876), the phosphors prepared in this work are all of Sr4Al14O25 and the doped Eu2+ have little influence on the structure of luminescence materials.
Figure 3 shows the FE-SEM of the 4Sr0.98Eu0.02O·7Al2O3 powders calcined at 1350°C for 3 h. It was observed that the microstructure of the phosphor consisted of irregular fine grains with an average size of about 8–10 μm.
The photoluminescence spectra of 4Sr0.98Eu0.02O·7Al2O3 measured at room temperature were shown in Fig. 4. As seen from Fig. 4, there was a broad excitation band ranged from 300 nm to 450 nm, which means this phosphor may be suitable for application on white LEDs excited by violet light. The typical excitation spectrum of Eu2+ is attributed to transition from 4f 7 ground state to the excited sate 4f 65d 1. The NUV-excited 4Sr0.98Eu0.02O·7Al2O3 phosphor (λ ex = 367, 397 nm) at room temperature yielded a very strong blue–green emission band peaked at 491 nm, which attributes to a typical 4f 65d 1→4f 7 transition of Eu2+. However, there were two emission bands: a main band peaked at 491 nm and a weak peak at 424 nm, when the concentration of doped-Eu2+ was less than 1 mol% as shown in Fig. 5. This is due to the presence of two different crystallographic sites for Sr2+ ions in the Sr4Al14O25 host (see Fig. 1) [23–26]. Because the radius of Sr2+ (0.114 nm) is very similar to that of Eu2+ (0.112 nm) [27], the doped Eu2+ ions substitute Sr2+ sites, leading to two types of Eu2+ sites in the Sr4Al14O25 crystals [26, 28, 29]. Although both sites have the same abundance, the 491 nm emission is, by far, the dominating emission. With increasing concentration of doped Eu2+ ions, the emission intensity of Eu2+ at 424 nm decreases and completely disappears as the concentration increases up to 1.5 mol%, while the 491 nm emission intensity increases until a maximum intensity is reached, and then decreases due to concentration quenching. From Fig. 5, we can see that the critical quenching concentration of Eu2+ in Sr4Al14O25:Eu2+ phosphor is about 2.0 mol%. This favorable behavior is due to the efficient energy transfer from the 424 nm emitting Eu2+ ions to the 491 nm emitting Eu2+. The emission peaked at 424 nm overlaps with the excitation spectra for the phosphor samples. Thus, the emitting light energy can be reabsorbed, which leads to an enhancement in the emission of 491 nm, but to a decrease in the emission at 424 nm.
Our purpose is to obtain a highly efficient light-conversion phosphor for NUV-LED, so a blue–green light-emitting LED was fabricated with 4Sr0.98Eu0.02O·7Al2O3 as phosphor. The emission spectrum of the fabricated LED is shown in Fig. 6. A strongly broad emitting band peaked at 493 nm and a weakly narrow emitting band peaked at 611 nm, which can attribute to 4f → 4f transition of the trace remains of Eu3+, appear excited by InGaN NUV chip while the 397 nm emitting peak from the chip itself partly remains. The CIE coordinates of the LED are X = 0.176 and Y = 0.412. From the standpoint of application, each proper phosphor must meet the following necessary conditions [30]. Firstly, the phosphor must efficiently absorb the 400 nm excitation energy that InGaN chip emitted. Secondly, the phosphor exhibits higher luminescent intensity under ∼400 nm excitation. Thirdly, the stability of the phosphor is high enough. Since Sr4Al14O25:Eu2+ meets all these conditions, it is consider to be a good blue–green phosphor for white LED excited with NUV LED chip.
4 Conclusions
Sr4Al14O25:Eu2+ phosphor was prepared by the solid-state reaction at 1350°C for 3 h in a reductive atmosphere of active carbon. The phosphor is efficiently excited by NUV-violet light from 300 nm to 450 nm and exhibits bright blue–green emission. A bright blue–green LED was fabricated by incorporating the phosphor with an InGaN NUV chip. All the characteristics indicate that Sr4Al14O25:Eu2+ is a good candidate phosphor applied in white LEDs.
References
S. Nakamura, M. Senoh, T. Mukai, Appl. Phys. Lett. 62, 2390 (1993)
S. Nakainura, G. Fasol, in The Blue Laser Diode:GaN Based Light Emitters and laser (Springer, Berlin, 1997)
P. Schlotter, J. Baur, Ch. Hielscher, M. Kunzer, H. Obloh, R. Schmidt, J. Schneider, Mater. Sci. Eng. B 59, 390 (1999)
T. Tamura, T. Setomoto T. Taguchi, J. Lumin. 87–89, 1180 (2000)
S. Nakamura, Appl. Phys. Lett. 64, 1687 (1994)
S. Aanegola, J. Petroski, E. Radkov, SPIE 10, 16 (2003)
Y. Narukawa, Opt. Photonics News 4, 25 (2004)
Y.S. Hu, W.D. Zhuang, H.Q. Ye, S.S. Zhang, J. Lumin. 111, 139 (2005)
C.F. Guo, C.X. Zhang, Y.H. Lu, Q. Tang, Q. Su, Physia Status Solidi A-Appl Res 201, 1588 (2004)
X.M. Zhang, J.H. Zhang, J. Xu, Q. Su, J. Alloys Comp. 389, 247 (2005)
J. Zhang, M. Takahashi, Y. Tokuda, T. Yoko, J. Ceramic Soc. Jap. 112, 511 (2004)
H. Wu, X.M. Zhang, C.F. Guo, J. Xu, M.M. wu, Q. Su, IEEE Photonics Technol Lett 17, 1160 (2005)
J.K. Park, M.A. Lim, C.H. Kim, H.D. Park, J.T. Park, S.Y. Choi, Appl. Phys. Lett. 82, 683 (2003)
J.S. Kim, P.E. Jeon, J.C. Choi, H.L. Park, S.I. Mho, G.C. Kim, Appl. Phys. Lett. 84, 2931 (2004)
J.S. Kim, P.E. Jeon, Y.H. Park, J.C. Choi, H.L. Park, G.C. Kim, T.W. Kim, Appl. Phys. Lett. 85, 3696 (2004)
S. Neeraj, N. Kijima, A.K. Cheetham, Chem. Phys. Lett. 387, 2 (2004)
B. Smets, J. Rutten, G. Hoeks, J. Electrochem. Soc. 136, 2119 (1989)
F.C. Palilla, A.K. Levine, M.R. Tomkus, J. Electrochem. Soc. 115, 642 (1968)
Y.H. Lin, Z.T. Zhang, F. Zhang, Z.L. Tang, Q.M. Chen, Mater. Chem. Phys. 65, 103 (2000)
V. Abbruscato, J. Electrochem. Soc. 118, 930 (1971)
Z.C. Wu, J.X. Shi, J. Wang, H. Wu, Q. Su, M.L. Gong, Mater. Lett. 60, 3499 (2006)
T.N. Nadzhina, E.A. Pobedimskaya, N.V. Belov, Kristallografiya 25, 938 (1980)
M.Q. Wang, D. Wang, G.J. Lü, Mater. Sci. Eng. B 57, 18 (1998)
D. Wang, M.Q. Wang, G.J. Lü, J. Mater. Sci. 34, 4959 (1999)
M. Capron, F. Fayon, D. Massiot, A. Douy, Chem. Mater. 15, 575 (2003)
Y. H. Lin, Z.L. Tang, Z.T. Zhang, Mater. Lett. 51, 14 (2001)
G. Blasse, in Luminescence of Inorganic Solids (Plenum Press, New York, 1978)
Y.H. Lin, Z.L. Tang, Z.T. Zhang, C.W. Nan, Appl. Phys. Lett. 81, 996 (2002)
M.Y. Peng, Z.W. Pei, G.Y. Hong, Q. Su, Chem. Phys. Lett. 371, 1 (2003)
Z.L. Wang, H.B. Liang, L.Y. Zhou, M.L. Gong, Q. Su, Chem. Phys. Lett. 412, 313 (2005)
Acknowledgements
This work was financially supported by grants from the Guangdong Province Government (ZB2003A07), the Natural Science Foundation of Guangdong Province (No.021716), and the Science and Technical Projects of Guangzhou (054J205001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Z., Shi, J., Wang, J. et al. Synthesis and luminescent properties of Sr4Al14O25:Eu2+ blue–green emitting phosphor for white light-emitting diodes (LEDs). J Mater Sci: Mater Electron 19, 339–342 (2008). https://doi.org/10.1007/s10854-007-9325-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-007-9325-2