Abstract
Modified Aurivillius-type-structured piezoelectric ceramics, Li0.04Ce0.04Na(0.46−x/2)Bi(4.46+x/2)Ti(4−x)Sc x O15 (LiCe–NBT–Sc−x, x = 0, 0.025, 0.075, 0.125, 0.15, 0.175) were synthesized by using conventional solid-state reaction process. Rietveld refinement for the x = 0.125 modified sample was carried out by using powder X-ray diffraction and LiCe–NBT–Sc−0.125 was confirmed to be a four-layer Aurivillius oxide with orthorhombic space group A2 1 am [a = 5.45814(7) Å, b = 5.43029(7) Å, c = 40.8547(4) Å and V = 1,210.902(26) Å3; Z = 4], at room temperature. The Sc3+/Bi3+ substitution led to an increase in Curie temperatures (T c) and an enhancement in piezoelectric property, and the LiCe–NBT–Sc−0.125 ceramic with a T c of 675 °C had a high piezoelectric activity (d 33) of 32 pC/N. Variable thermal-annealing d 33 and resistivity (ρ) of the LiCe–NBT–Sc−0.125 ceramic were investigated; the d 33 of the O2 annealed sample reached up to 37 pC/N, and its resistivity was about 6.8 × 106 Ω cm at 575 °C and about 6.5 × 105 Ω cm at 650 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aurivillius ferroelectrics (bismuth layer structure ferroelectrics, BLSFs) are thought to be promising materials for sensors, actuators, and nonvolatile ferroelectric random access memories (NvFRAMs), owing to their fatigue-free properties and relatively high Curie temperature (T c) [4, 25, 26, 36]. The general formula of BLSFs is (Bi2O2)2+(Am−1BmO3m+1)2−, where A is a mono-, di- or trivalent element (or a combination of them) allowing dodecahedral coordination, e.g., Ca2+, Sr2+, Ba2+, Pb2+, Bi3+, K+, Na+; B is a transition element suited to octahedral coordination, like Nb5+, Ta5+, Ti4+, Mo6+, W6+ or V5+. m is the number of octahedral layers in the perovskite layers and its values vary from 1 to 6 [1–3, 33]. BLSFs are thought to be the best candidates for high-temperature and ultrahigh-temperature piezoelectric applications [16, 31, 36, 37, 43], due to their high Curie points (T c) and high temperature stablities. Nevertheless, the piezoelectric coefficient (d 33) of most Aurivillius ferroelectrics with high T c (T c > 650 °C) is relatively low, with values ≤20 pC/N [29, 36, 37, 39, 40], owing to their large coercive fields (E c) and their structural anisotropy.
It is reported that the A-site co-substitution of alkali metal cation (Li+, K+, Na+, etc.) and lanthanon cation (La3+, Ce3+, Nd3+, Pr3+, etc.) can improve the piezoelectric properties and ferroelectric properties of the BLSFs effectively, especially the co-substitution of Li+ and Ce3+ [10, 11, 35]. According to Gai et al. and H. Yan et al. [11, 35] with (Li,Ce) A-site co-substitution, [(Na0.52K0.42Li0.06)Bi]0.44(LiCe)0.03[]0.03Bi2–(Nb1.88Sb0.06Ta0.06)O9 and Ca0.85(Li,Ce)0.075Bi4Ti4O15 had high piezoelectric activities (d 33) and low dielectric losses (tanδ), which is considered to be owing to the enhancement of grain growth and lattice distortion. In addition, the effect of the Sc3+ substitution with the largest d0 size 0.745 Å, six coordination number (6 CN) on ferroelectric polarization and piezoelectric activity is much effective. The Aurivillius oxides Na0.5−x Bi2.5+x Nb2−x Sc x O9 prepared in our previous work and Bi2+2x Sr1−2x Ta2−x Sc x O9 prepared by Sivakumar et al. both showed a significant enhancement in ferroelectric and piezoelectric properties. Also, the Sc3+ substitution at the B site give rise to an obvious elevation in ferroelectric to paraelectric phase transition temperatures (T c) [22, 27], which is most important for piezoelectrics in high-temperature applications.
Na0.5Bi4.5Ti4O15 (NBT) is one member of four-layer-structured Aurivillius phase oxides and has high T c and outstanding piezoelectric and electromechanical properties [24]. According to Wang et al. [32] with 0.04 LiCe substitution for the A-site NaBi, the Li0.04Ce0.04Na0.5Bi4.5Ti4O15 ceramics with a T c of about 660 °C had relatively high d 33 of 30 pC/N and high temperature stability, which suggests that Li0.04Ce0.04Na0.5Bi4.5Ti4O15 is a promising high-temperature piezoelectric material. In the present work, we prepared new Aurivillius oxides Li0.04Ce0.04Na(0.46−x/2)Bi(4.46+x/2)Ti(4−x)Sc x O15 (LiCe–NBT–Sc−x, x = 0, 0.025, 0.075, 0.125, 0.15 and 0.175 abbreviated as LiCe–NBT, LiCe–NBT–Sc−0.025, LiCe–NBT–Sc−0.075, LiCe–NBT–Sc−0.125, LiCe–NBT–Sc−0.15 and LiCe–NBT–Sc−0.175, respectively) by using a solid-state reaction process, where Sc3+ substituted the B-site Ti4+ and the A-site Na+ was replaced by the high valence state Bi3+ for the sake of the charge compensation in oxides as well as an enhancement in piezoelectric property. The structures, dielectric properties, piezoelectric properties and electrical conduction of the materials were studied in detail to clarify the effect of Sc3+ substitution.
2 Experimental
Sodium bismuth titanate piezoelectric ceramics with A-site Li and Ce co-substitution and B-site Sc3+ substitution were prepared by the standard ceramic fabrication procedure. The starting materials used were Bi2O3 (99 %), Na2CO3 (99.8 %), TiO2 (99 %), Li2CO3 (99.99 %), CeO2 (99.99 %) and Sc2O3 (99.99 %). The powders were mixed at a desired weight ratio and ball-milled in ethanol with zirconia balls for 24 h followed by calcination at 800 °C for 2 h in an alumina crucible. The ground calcinated powders were ball-milled again for 24 h and dried, and then uniaxially formed into pellets of 12 mm in diameter and 1 mm in thickness. These pellets were then cold isostatically pressed at 300 MPa to obtain dense green specimens and then were fully surrounded by the powder of matching compositions in a closed alumina crucible and sintered at 1,065 °C for 3 h. The size of the final samples is about 8.5 mm in diameter and 0.5 mm in thickness. Silver electrodes were baked on the opposite faces of the samples and fired at 850 °C for 30 min to form a metal–insulator-metal capacitor for electrical test.
Powder X-ray diffraction (XRD) data were collected by using an automated diffractometer (X’Pert PRO MPD, Philips, Eindhoven, The Netherlands) with a nickel filter (Cu-Ka radiation) at room temperature. Surface morphologies of the ceramics were examined by a scanning electron microscope (SEM; JSM-5610, JEOL, Tokyo, Japan). Raman spectra were obtained with an instrument (LabRAM HR800, Horiba Jobin–Yvon, Lyon, France) in a backward scattering geometry (the exciting source was the 514.5 nm line from an argon ion laser). High-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) images of the ceramics were observed by using transmission electron microscopy (TEM; Tecnai F30, FEI, Hillsboro, OR, USA) operated at 300 kV accelerating voltage. Temperature dependence of dielectric properties of the samples was measured by using an LCR meter (4284A, Agilent, CA, USA). After samples were poled in silicone oil under a dc electric field of 100–130 kV cm−1 for 30–40 min at 160 °C, their piezoelectric coefficients (d 33) were determined by using a piezo-d 33 meter (ZJ-3B, Institute of Acoustics, Chinese Academic of Science, Beijing, China). Variable-temperatures impedance spectroscopies (IS) of the ceramics were performed by using an Impedance Analyzer (SI1260, Solartron, Hampshire, UK) in the frequency range from 1 Hz to 1 MHz.
3 Results and discussion
3.1 Crystal structure and phase
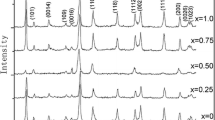
XRD patterns of all LiCe–NBT–Sc−x ceramics are shown in Fig. 1a, and no second phase is found. It means that single phase was obtained for all samples, and Li+/Ce3+/Sc3+ was incorporated into the crystalline structure. The peak associated with the (119) plane has the highest intensity, indicating that the ceramics have the bismuth layered structure with m = 4. With an increase of x, all the diffraction peaks of the LiCe–NBT–Sc−x shift toward lower 2θ angle positions, especially for these peaks showed in the insert of Fig. 1a. Like Bi3+/Sc3+ substituted Na0.5−x Bi2.5+x Nb2−x Sc x O9 ceramics [22], the low shifts of the diffraction peaks should be attributed to the facts that the Bi3+/Sc3+ substitution for A- and B-site Na+/Ti4+ leads to an increase in lattice parameters and an enlargement in unit cell volume, because of the larger size of Sc3+ (0.745 Å, 6 CN) compared to that of Ti4+ (0.605 Å, 6 CN). Surfaces SEM image of LiCe–NBT–Sc−0.125 ceramic is shown in the inset of Fig. 1b. The plate-like morphology is observed for it, which is typical characteristic of Aurivillius ceramics due to the anisotropic nature of crystal structure.
Raman spectra of LiCe–NBT–Sc−x ceramics in the range from 100 to 1,000 cm−1 are shown in Fig. 1b. For Raman spectrum of bismuth layer structured crystals, it is generally recognized that their phonon modes can be classified into two categories: low frequency modes below 200 cm−1 and high frequency modes above 200 cm−1. The low frequency modes below 200 cm−1 are related to the cation vibrations in the perovskite layers, while the high frequency modes above 200 cm−1 primarily come from the torsional bending and the stretching modes of BO6 octahedral [8, 19]. It is seen that the Raman spectra of LiCe–NBT–Sc−x have six intensive modes at around 118, 269, 339, 548, 570 and 857 cm−1. The positions of peak near 118, 150 and 259 cm−1 are ascribed to the vibration of A-site ions. It is easily to be found that the peak at 118 cm−1 shifted slightly towards higher frequency and the relative intensity of peak at 150 cm−1 gradually decreased with increasing x, which is due to the variation of A-site Na+ and Bi3+ content. Since the peak at 857 cm−1 is caused by the streching of TiO6 octahedral [17], and we find that the peaks at 339, 718 and 857 cm−1,shifted slightly towards higher frequency with increasing x, which indicates that Sc3+ had been diffused into the LiCe–NBT crystal structure and caused the torsional bending of TiO6 octahedral.
In order to characterize structures of the LiCe–NBT–Sc−x oxides, XRD Rietveld refinement for the representative LiCe–NBT–Sc−0.125 was carried out with A21 am space group at room temperature, by using the GSAS-EXPGUI program [18, 22, 28]. The XRD data were collected in 2θ range of 10°–120° with a step size of 0.008° and a step time of 4 s. The coordinates of SrBi4Ti4O15 [13] were used as an initial model where Bi (52.25 %), Na (39.75 %), Li (4 %) and Ce (4 %) co-occupied the Bi1/Sr1 (4a) site, Bi (100 %) occupied the Bi2/Sr2 (8b) site, and Sc (6.25 %) occupied the Ti1 (8b) site, respectively. In the refinement process, the occupancies of the Bi and Na atoms between A1 and A2 sites were released. Also, it is reasonable that Sc can substitute Ti and occupy in the Ti2 (8b) site. However, the occupancies of the Li and Ce atoms were not refined and fixed to be 4 % due to their low level substitution. Figure 2 shows that the final refined profile of the LiCe–NBT–Sc−0.125 fit well its XRD data, and the final factors, R wp, R p and Chi squared values (χ2) are 6.45, 4.75 and 2.76 %, respectively. The refined lattice parameters of the LiCe–NBT–Sc−0.125 are a = 5.45814(7) Å, b = 5.43029(7) Å, c = 40.8547(4) Å and V = 1,210.902(26) Å3. Table 1 lists the atomic coordinates, atomic occupancies and isotropic thermal parameters of the LiCe–NBT–Sc−0.125. On the Basis of the refined results, the crystal structures of the LiCe–NBT–Sc−0.125 viewed along [100], [\({\bar{\text{1}}\text{ 1}}0\)] and [010] directions at room temperature are depicted in Fig. 3a. Figure 3b shows a HRTEM image of the the LiCe–NBT–Sc−0.125 viewed along the [\({\bar{\text{1}}\text{ 1}}0\)] zone-axis. The periodic fringes corresponding to the (Bi2O2)2+ layers are clearly observed, with a spacing of about 2 nm equivalent to half of the lattice parameter c of the LiCe–NBT–Sc−0.125, 4.08547(4) nm. The corresponding SAED pattern recorded along the [\({\bar{\text{1}}\text{ 1}}0\)] zone axis is shown in Fig. 3c. This pattern can be indexed according to an A-centered orthorhombic cell (A21 am) with the refined lattice parameters of the LiCe–NBT–Sc−0.125.
3.2 Effect of the Sc3+/Bi3+ substitution on properties
Figure 4 shows temperature dependence of the dielectric permittivity (ɛ′) and the dielectric loss (tanδ) of all samples at 1 MHz. The Curie point of LiCe–NBT is 649 °C, which is close to that reported by Wang el at. and an increase in x value leads to an increase in T c. The T c values of LiCe–NBT–Sc−0.125 and LiCe–NBT–Sc−0.175 ceramics are around 675 and 680 °C, respectively. The ferroelectric to paraelectric phase transition temperature of the prepared samples should be closely related to their structural deformation, and the Bi3+/Sc3+ substitution for A- and B-site Na+/Ti4+ could cause an enhancement in orthorhombic distortion. Commonly, this distortion is explained in terms of a lattice mismatch between BO2 and AO planes in the perovskite-type unit of AB2O7, and the tolerance factor (t) of the perovskite-layer units is given by [23]
In Eq. (1), r A , r B and r O are ionic radii of an A-site cation, a B-site cation, and an oxygen ion, respectively. A lower t value often accompanies a higher T c. It is obvious that the B-site Sc3+ substitution for Ti4+ has a significant effect on T c, which can be ascribed to the decrease of t, because the size of Sc3+ (0.745 Å, 6 CN) is larger than that of Ti4+ (0.605 Å, 6 CN). Since the anisotropic behavior of the A-site Bi3+ cation with 6s2 lone pair electrons causes the deformation from the prototype structure [9], the replacement of the non-polar Na+ by the polar Bi3+ would aggravate the orthorhombic distortion and could further give rise to an increase in T c of the modified LiCe–NBT–Sc−x ceramics. Notably, an increase in Bi3+/Sc3+ substitution concentration also induces a decrease in the height of their dielectric permittivity peak decreased. In Fig. 4b, the losses (tanδ) of the Bi3+/Sc3+ substituted samples are lower than that of the LiCe–NBT in the temperature range of 30–400 °C. When the temperature is higher than 400 °C, the modified samples have higher tanδ values, which could be associated with a higher concentration of charge carriers at higher temperatures due to the Bi3+/Sc3+ substitution [6]. The loss peaks of all ceramics locate at about 620 °C and their heights are in the range from 0.17 to 0.51. With further increasing temperature, each loss peak for all samples falls into a valley, which is corresponding to the dielectric permittivity peaks and is the T c [41].
High-temperature resistivity is very important for Aurivillius piezoelectric high temperature applications. Figure 5a–c shows the impedance spectra of the LiCe–NBT, LiCe–NBT–Sc−0.125 and LiCe–NBT–Sc−0.175 ceramics over 575–750 °C. Normally, the electrode, grain boundary and grain components contribute to the conductivities of the polycrystal ceramics and their effects on the conduction can be segregated into three parallel RC elements connected in series on an equivalent circuit, from low frequency to high frequency [15]. For each sample, one Debye-like semicircle at high frequency and a little squashed semicircle at low frequency can be observed at variable temperature. The major Debye-like semicircle is in response to the grain effect and the weak small arc is attributed to the grain boundary effect, and the magnitude of the conductivity is predominantly determined by the classic bulk response. According to the grain semicircles, logarithmic resistivities (ρ) of all the LiCe–NBT–Sc−x ceramics are shown in Fig. 5. It is clear that the Sc3+/Bi3+ substitution leads to an increase in resistivity for the modified samples by and large, exception for the abnormal low resistivities of the x = 0.025 modified sample. Meaningfully, the LiCe–NBT–Sc−0.125 ceramic has relatively high resistivity of about 106 Ω cm at 575 °C and about 105 Ω cm at 650 °C, which would warrant its applicability for high-temperature piezoelectric devices. In addition, the activation energies (E a ) of all samples for electrical conduction can be detected by using the Arrhenius equation,
where E a is the nominal activation energy per charge carrier and k is Boltzmann’s constant. From Eq. (2), it can be seen that log ρ has a linear relationship with 1,000/T and theE a can be determined in terms of the slope of the Arrhenius plots. With the increase of Sc3+/Bi3+ substitution concentration, theE a values monotonously increased from 1.06 eV at LiCe–NBT to 2.04 eV at LiCe–NBT–Sc−0.175 [inset of Fig. 5d]. It is believed that the migration of defects (e.g., oxygen vacancies) in distorted structure is more difficult than in undistorted structure. The introducing of the Sc3+/Bi3+ in the modified LiCe–NBT–Sc−x samples led to an enhancement in lattice distortion, and their orthorhombic deformation is reinforced. Therefore, an increase in ρ and E a is observed in the substituted samples, and higher ρ and E a values is accompanied by higher substitution concentration. Additionally, the charged centrals (i.e., Bi ··Na and ScTi′) could attract some defects, e.g., holes (h ·) or oxygen vacancies (V ··O ), and suppress their movements. As a result, a further increase in ρ and E a is induced by the Sc3+/Bi3+ substitution.
The piezoelectric activity of the pure LiCe–NBT ceramic is 17 pC/N, and the Sc3+/Bi3+ introducing gives rise to an increase in d 33 of the modified LiCe–NBT–Sc−x samples. The enhancement of their piezoelectric activities can be attributed to the Sc3+ ions substitution for B-site Ti4+ ions. As the radium of Sc3+ ion is larger than Ti4+, the substitution of Sc3+ caused an enhancement in the distortion of the perovskite layers and a corresponding increase in ferroelectric spontaneous polarization (P s), due to the A-site polar ion and B-site large size Sc3+ ion co-substitution [12]. The modified LiCe–NBT–Sc−0.125 sample has the highest d 33 of 32 pC/N, which is also higher than the d 33 values of other ordinary sintered BLSF ceramics. However, a further increase of concentration of Sc3+/Bi3+ could cause a dimensional mismatch between perovskite and bismuth oxide layers, and the presence of the charged defect cations (i.e., Bi ··Na and ScTi′) could act as pinning centers for domain walls and make domains’ reversal difficult. In consequence, a significant decrease in d 33 is observed in the LiCe–NBT–Sc−0.15 and LiCe–NBT–Sc−0.175 samples, and their d 33 values are 25 and 23 pC/N. This is consistent with the previous reports that the addition of Nb and other cations in perovskite layer [7, 14]. Figure 6a shows the effect of thermal depoling on the piezoelectric activities of all samples. The d 33 values were measured at room temperature after annealing for 2 h at each temperature (corresponding to the x-axis temperature). Notably, all sample have 80 % of their d 33 values at room temperature value even if the annealing temperature approaches the T c, and the LiCe–NBT–Sc−0.125 sample still has a high d 33 of 25 pC/N after 650 °C thermal annealing. This reveals that the piezoelectric properties of the LiCe–NBT–Sc−x ceramics are stable against temperature. For each sample, the unstable piezoelectric activity after thermal treatment could be contributed from the movements of non-180° domain walls [37, 38]. For all samples, the d 33 decreases rapidly when the annealing temperature is near T c, and tends to zero when the temperature is increased above T c.
3.3 Thermal annealing behaviors of the d 33 and ρ
To further enhance the piezoelectric activity, the LiCe–NBT–Sc−0.125 sample was annealed with different atmospheres (O2, air and N2) at 800 °C for 2 h. Interestingly, a significant increase in d 33 is observed for the O2 annealed sample, and its d 33 value reaches up to 37 pC/N, which is higher than those of the state-of-the-art Aurivillius piezoelectric ceramics with high T c (T c ≥ 600 °C). This increased d 33 should be attributed to the fact that the O2 annealing induces a weak in the defect (mainly oxygen vacancies) “pinning” on the domain walls and an increase in the number of available switching ferroelectric domains. Whereas, the air and N2 annealed samples have lower d 33 as compared to that of the un-annealed sample. After annealed in O2, the pinning effects caused by oxygen vacancies and internal stress on domain wall motion were largely reduced or eliminated, which made the samples easier to be poled and lead to higher d 33 values [30, 34, 42]. While the air and N2 annealings could cause an increase in the number of oxygen vacancies, and the defect (mainly oxygen vacancies) “pinning” on the domain walls is reinforced correspondingly; thus, the poling became harder and the piezoelectric activity was reduced. Also, the d 33 of these annealed samples against temperature is shown in Fig. 6b. It is observed that the d 33 change trend of the O2 annealed sample is consistent with that of the un-annealed sample, and the sample still has a high d 33 of 30 pC/N at 650 °C. Comparatively, an improvement in piezoelectric temperature stability is observed for the air and N2 annealed samples. It is likely that more charged defects and defect dipoles (e.g., ScTi′–V ··O ) owing to the oxygen loss, interact with non-180° domain walls and “freeze” their response [5]. Therefore, the domain-wall contribution can be “frozen”, which leads to relatively more stable piezoelectric properties of the air and N2 annealed samples.
Figure 7 shows variable-temperature impedance spectra of the O2 annealed LiCe–NBT–Sc−0.125 ceramic. Like the un-annealed sample, each Z* plot of the O2 annealed at each temperature contains one Debye-like semicircle at high frequency associated with the grain effect and a little squashed semicircle at low frequency in response to the grain boundary effect, and the magnitude of the total resistivity is mainly determined by the grain response. Differently, the O2 annealing results in an increase in size of the Debye-like semicircle. Determined from the sizes of these impedance semicircles, variable-temperature logarithmic resistivities (grain effect) of the O2 annealed sample are shown in the inset of Fig. 7. It is observed that the bulk resistivity of the O2 annealed sample is higher than that of the un-annealed sample at each temperature, and has much high resistivity of about 6.8 × 106 Ω cm at 575 °C and about 6.5 × 105 Ω cm at 650 °C. Correspondingly, the E a of the O2 annealed sample is 1.94 eV, which is higher that of the un-annealed sample (1.88 eV). However, the N2 annealing causes the bulk resistivities of the LiCe–NBT–Sc−0.125 ceramic decreasing at variable temperatures, and this E a value is decreased to 1.82 eV. In the present LiCe–NBT–Sc−x ceramics, some cations (Bi3+, Na+) could volatilize during sintering and cation vacancies possibly exist at the lattices, which resulted in oxygen vacancies due to charge neutrality restriction [20, 21]. Consequently, some oxygen vacancies exist in the lattice positions. The O atoms from the O2 atmosphere can be diffused into the V ··O sites, and a decrease in the V ··O concentration is induced by the O2 annealing, while the N2 annealing could lead to an increase in the number of defects (V ··O ) and a decrease in E a due to a further oxygen loss.
4 Conclusions
Modified Aurivillius piezoelectric ceramics, Li0.04Ce0.04Na(0.46−x/2)Bi(4.46+x/2)Ti(4−x)Sc x O15 (LiCe–NBT–Sc−x, x = 0, 0.025, 0.075, 0.125, 0.15, 0.175) were synthesized by using a traditional solid-state reaction process, where Sc3+ substituted B-site Ti4+ and excess Bi3+ substituted A-site Na+. The structures and domain structures of the prepared ceramics were characterized by using XRD, SEM HRTEM, SAED and Raman spectroscopy. The crystal structure refinement and Raman spectra of the LiCe–NBT–Sc−x verified that Sc3+ and excess Bi3+ were incorporated into the perovskite lattice, and they occupied the B-site and A-site, respectively. The refined lattice parameters of the LiCe–NBT–Sc−0.125 are a = 5.45814(7) Å, b = 5.43029(7) Å, c = 40.8547(4) Å, at room temperature. The modified samples showed elevated T c due to the Sc3+/Bi3+ substitution, and their T c value is increased from 649 °C of pure Li–NBT to 680 °C. The LiCe–NBT–Sc−0.125 ceramic with an appropriate level modification (x = 0.125) had the highest piezoelectric activity (d 33) and the highest resistivity, with a T c of 675 °C. After O2 atmosphere annealing, a further increase in d 33 is obtained for the LiCe–NBT–Sc−0.125 ceramic, and this d 33 value reached up to 37 pC/N, which is higher than those of the state-of-the-art Aurivillius piezoelectric ceramics with high T c (T c ≥ 600 °C). Meaningfully, this piezoelectric activity was stable against temperature, and the O2 annealed LiCe–NBT–Sc−0.125 ceramic still had a relatively high d 33 of 30 pC/N at 650 °C. In addition, its resistivity was of about 6.8 × 106 Ω cm at 575 °C and about 6.5 × 105 Ω cm at 650 °C.
References
B. Aurivillius, Ark. Kemi. 1, 463–499 (1949)
B. Aurivillius, Ark. Kemi. 2, 519 (1950)
B. Aurivillius, Ark. Kemi. 5, 39 (1952)
X.F. Cao, L. Zhang, X.T. Chen, Z.L. Xue, CrystEngComm 13, 306–311 (2011)
D. Damjanovic, M. Demartin, H.S. Shulman, M. Testorf, N. Setter, Sens. Actuators, A 53, 353–360 (1996)
H.L. Du, X. Shi, J. Phys. Chem. Solids 72, 1279–1283 (2011)
H.C. Du, L.J. Tang, S.J. Kaskel, Phys. Chem. C 113, 1329–1339 (2009)
P.Y. Fang, H.Q. Fan, Z.Z. Xia, W.X. Chen, Solid State Commun. 152, 979–983 (2012)
B. Frit, J.P. Mercurio, J. Alloys Comp. 188, 27–35 (1992)
Z.G. Gai, J.F. Wang, C.M. Wang, Appl. Phys. Lett. 90, 0529111–0529113 (2007)
Z.G. Gai, Y.Y. Feng, J.F. Wang, M.L. Zhao, L.M. Zheng, C.M. Wang, S.J. Zhang, T.R. Shrout, Phys. Status Solidi A 8, 1792–1795 (2010)
D.J. Gao, K.W. Kwok, D. Lin, Curr. Appl. Phys. 11, S124–S127 (2011)
C.H. Hervoches, A. Snedden, R. Riggs, S.H. Kilcoyne, P. Manuel, P. Lightfoot, J. Solid State Chem. 164, 280–291 (2002)
J.G. Hou, R.V. Kumar, Y.F. Qu, D. Krsmanovic, Scr. Mater. 61, 664–667 (2009)
J.T.S. Irvine, D.C. Sinclair, A.R. West, Adv. Mater. 2, 132–138 (1990)
L.B. Kong, T.S. Zhang, J. Ma, Y.C.F. Boey, Prog. Mater Sci. 53, 207–322 (2008)
W.J. Kuai, M.L. Zhao, C.L. Wang, L.H. Wang, J. Wang, Z.G. Gai, J.L. Zhang, N. Yin, J. Alloys Comp. 559, 76–80 (2013)
A.C. Larson, R.B. Von Dreele, General Structure Analysis System (GSAS), LANL Report LAUR 86–748 (Los Alamos National Laboratory, Los Alamos, 2000)
J.J. Liu, G.T. Zou, Y.R. Jin, J. Phys. Chem. Solids 51, 1653–1658 (1996)
C.B. Long, H.Q. Fan, Dalton Trans. 41, 11046–11054 (2012)
C.B. Long, H.Q. Fan, M.M. Li, Q. Li, CrystEngComm 14, 7201–7208 (2012)
C.B. Long, H.Q. Fan, M.M. Li, Q. Li, Eur. J. Inorg. Chem. 32, 5622–5630 (2013)
C.B. Long, H.Q. Fan, M.M. Li, P.R. Ren, Y. Cai, CrystEngComm 15, 10212–10221 (2013)
R.E. Newnham, Mater. Res. Bull. 2, 1041–1044 (1967)
B.H. Park, B.S. Kang, S.D. Bu, T.W. Noh, J. Lee, W. Jo, Nature 401, 682–684 (1999)
C.A. Paz de Araujo, J.D. Cuchiaro, L.D. McMillan, M.C. Scott, J.F. Scott, Nature 374, 627–629 (1995)
T. Sivakumar, M. Itoh, J. Mater. Chem. 21, 10865–10870 (2011)
B.H. Toby, J. Appl. Crystallogr. 34, 210–213 (2001)
M. Villegas, T. Jardiel, G.J. Farias, Eur. Ceram. Soc. 24, 1025–1029 (2004)
N. Vittayakorn, D.P. Cann, G. Rujijanagul, T. Tunkasiri, Ferroelectrics 348, 94–105 (2007)
C.M. Wang, J.F. Wang, Z.G. Gai, Scr. Mater. 57, 789–792 (2007)
C.M. Wang, J.F. Wang, S.J. Zhang, Thomas R. Shrout. J. Appl. Phys. 105, 0941101–0941105 (2009)
Y. Wu, M.J. Forbess, S. Seraji, S.J. Limmer, T.P. Chou, C. Nguyen, G. Cao, J. Appl. Phys. 90, 5296–5302 (2001)
F. Xia, X. Yao, J. Appl. Phys. 92, 2709–2716 (2002)
H.X. Yan, Z. Zhang, W.M. Zhu, L.X. He, Y.H. Yu, Mater. Res. Bull. 39, 1237–1246 (2004)
H.X. Yan, H.T. Zhang, R. Ubic, M.J. Reece, J. Liu, Z. Shen, Z. Zhang, Adv. Mater. 17, 1261–1265 (2005)
H.X. Yan, H.T. Zhang, M.J. Reece, X.L. Dong, Appl. Phys. Lett. 87, 0829111–0829113 (2005)
H.X. Yan, H.T. Zhang, Z. Zhang, R. Ubic, M.J. Reece, J. Eur. Ceram. Soc. 26, 2785–2792 (2006)
J.T. Zeng, Y.X. Li, D. Wang, Q.R. Yin, Solid State Commun. 133, 553–557 (2005)
X.D. Zhang, H.X. Yan, M.J. Reece, J. Am. Ceram. Soc. 91, 2928–2932 (2008)
T.L. Zhao, Z.L. Guo, C.M. Wang, J. Am. Ceram. Soc. 95, 1062–1067 (2012)
Y.H. Zhen, J.F. Li, K. Wang, Y.G. Yan, L.Q. Yu, Mater. Sci. Eng. B-Solid 176, 1110–1114 (2011)
Z.Y. Zhou, X.L. Dong, H. Chen, J. Am. Ceram. Soc. 89, 1756–1760 (2006)
Acknowledgments
This work was supported by the National Natural Science Foundation (51172187), the SRPDF (20116102130002, 20116102120016) and 111 Program (B08040) of MOE, the Xi’an Science and Technology Foundation (CX1261-2, CX1261-3, CX12174, XBCL-1-08), the Shaanxi Province Science Foundation (2013KW12-02), the NPU Fundamental Research Foundation (NPU-FFR-JC201232), and the Excellent Doctorate Foundation of Northwestern Polytechnical University of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Fan, H., Long, C. et al. Crystal structure and thermal annealing behaviors of high d 33 Aurivillius-phase ceramics Li0.04Ce0.04Na(0.46−x/2)Bi(4.46+x/2)Ti(4−x)Sc x O15 with the Sc3+/Bi3+ co-substitution. J Mater Sci: Mater Electron 25, 2961–2968 (2014). https://doi.org/10.1007/s10854-014-1967-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-1967-2