Abstract
Thin films of copper selenide were deposited onto amorphous glass substrates at various substrate temperatures by computerized spray pyrolysis technique. The as deposited copper selenide thin films were used to study a wide range of characteristics including structural, surface morphological, optical and electrical, Hall Effect and thermo-electrical properties. X-ray diffraction study reveals that the films are polycrystalline in nature with hexagonal (mineral klockmannite) crystal structure irrespective of the substrate temperature. The crystalline size is found to be in the range of 23–28 nm. The SEM study reveals that the grains are uniform with uneven spherically shaped and spread over the entire surface of the substrates. EDAX analysis confirmed the nearly stoichiometric deposition of the film at 350 °C. The direct band gap values are found to be in the range 2.29–2.36 eV depending on the substrate temperature. The Hall Effect study reveals that the films exhibit p-type conductivity. The values of carrier concentration and mobility for the film are found to be 5.02 × 1017 cm−3 and 5.19 × 10−3 cm2 V−1 s−1; respectively for film deposited at 350 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Currently; much importance has been given to study the deposition and characterization of semiconducting metal chalcogenide and chalcopyrite thin films because of their various optoelectronic properties and applications. There are considerable interests in the field of solar selective coating, optoelectronic devices, electronics and electrical devices [1–7]. Copper selenide is a p-type semiconductor material due to copper vacancies; that has suitable electrical and optical properties for a number of applications in solar cells, super ionic conductors and photo-detectors, etc. The indirect band gap of 1.1–1.27 eV makes this material an absorbent material and a direct band gap >2 eV, makes it a window layer material in solar cells [8, 9].
Selenides of copper with different compositions, stoichiometric (α-Cu2Se, Cu3Se2, CuSe, and CuSe2) and non-stoichiometric (Cu2−xSe), and structural forms are well-documented [10]. CuSe is reported as hexagonal at room temperature and undergoes the transition to orthorhombic at the 48 °C and back to hexagonal at 120 °C [10]. At still higher temperatures, CuSe disproportionate into Cu2−xSe and selenium [10]. Among these phases, it has been demonstrated that the non-stoichiometric Cu2−xSe thin films has been used to form a junction with n-type semiconductors either as absorber in hetero-junction with CdS or as window layer material in hetero-junction. Chen et al. [11] developed a polycrystalline thin film solar cell back wall designed hetero-junction structure based upon p-type Cu2−xSe and n-type CdS semiconductor materials showing device efficiency of 5.38 % [11]. However, the works about copper selenide are relatively rare and the motivation of copper selenide thin film studies mainly focuses on application in the absorber layer of solar cell. In fact, in addition to application in solar cells, the copper selenide thin film could have unique electrical and optical performance [9].
Various methods so far adopted for the preparation of copper chalcopyrite thin films include chemical bath deposition [12], selenization [13, 14] sputtering [15], electrochemical deposition [16], galvanic synthesis [17], co-deposition [18], evaporation [19], electrodeposition [20, 21], solvothermal method [22], MOCVD [23] and spray pyrolysis technique etc. A very few reports are available on synthesis of copper selenide by spray pyrolysis. The spray pyrolysis method presents some noticeable advantages, such as: a wide possibility of varying the film properties by changing the composition of the starting solution (introduction of dopants and change the film microstructure) and low cost when large-scale production is needed. Since the study of optical and electrical analysis plays an important role in device fabrication (Opto-electronic devices). Keeping in view all these aspects an attempt has been made to deposit copper selenide thin films at various substrate temperatures and additionally its effect on optical, compositional and electrical properties has been studied.
2 Experimental details
The spray pyrolysis method is basically a chemical deposition technique in which fine droplets of the desired material are sprayed onto preheated substrates. Continuous films are formed onto the hot substrate by thermal decomposition of the material droplets. Copper selenide thin films were deposited onto glass substrates by a spray pyrolysis method at 300 to 400 °C substrate temperatures at the interval of 25 °C. Aqueous solutions (0.050 M) of copper chloride hydrate (CuCl2·2H2O) and selenourea (H2NC (Se) NH2) were utilized as starting materials. The various preparative parameters used were optimized with photoelectrochemical (PEC) techniques. Glass microslides of the size 7.5 × 2.5 cm2 were used as substrates. Before deposition these substrates were washed with water, then boiled in concentrated (2 M) chromic acid and kept in double distilled water for 48 h. Finally the substrates were ultrasonically cleaned for 10 min. The temperature of substrate was controlled by an iron–constantan thermocouple. A spray rate of 4 ml/min was kept constant throughout the experiment. The distance between the nozzle and the substrate was 28 cm. The air was used as carrier gas. After deposition, the films were allowed to cool at room temperature. The adhesion of the films onto the substrate was quite good.
The as deposited thin films of copper selenide were characterized for structural, morphological, compositional, optical, electrical, thermo-electric properties and Hall Effect measurements. The film thickness of the as-deposited films was measured by a well known gravimetric weight difference method using sensitive microbalance and assuming bulk density of CuSe. The structural characterization of the films was carried out by analyzing the X-ray diffraction (XRD) patterns obtained using Philips PW-3710 X-ray diffractometer with Cu-Kα radiation, within the 2θ range of angles between 10 and 80°. The surface morphology and compositional analysis of the spray deposited copper selenide thin films on glass substrate was carried out using JOEL–JSM 5600. Atomic force microscopy (AFM) was carried out in air at ambient condition (300 K) using Nanoscope III from Veeco. A UV–Vis spectrophotometer (SHIMADZU UV-1700) was used to record the optical absorption spectra of the samples in the wavelength range 350–850 nm. Electrical resistivity and thermo electric power measurements were done using D.C. two point probe method. Hall Effect setup supplied by Scientific Equipments, Roorkee, India was used for measurements of electrical parameters like carrier concentration (n) and mobility (μ) at room temperature. Van der Pauw technique [24] was used for this. Specially designed Hall probe on printed circuit board (PCB) was used to fix the sample of the size 10 × 10 mm2. Silver paste was employed to the films to ensure good electrical ohmic contacts.
3 Results and discussion
3.1 Growth of the films
Figure 1 shows the variation of film thickness versus substrate temperature; relatively higher thickness is found to be 546 nm for film deposited at 350 °C. It reflects that the film thickness increases with increasing substrate temperature from 300 °C to 350 °C, attain maximum value at 350 °C (546 nm), beyond that it decreases. This behavior can be explained as follows: Initially, at lower substrate temperatures e.g., 300 °C, the temperature may not be sufficient to decompose the sprayed droplets of Cu++ and Se− ions from the solution and this therefore, results in a lower thickness. At a particular substrate temperature 350 °C, decomposition occurs at the optimum rate resulting in the terminal thickness being attained. A noticeable decrease of the film thickness with increasing the substrate temperature is observed after substrate temperature 350 °C. This decrease may be attributed to re-evaporation of film material after deposition or to thermal convection of the sprayed droplet during the deposition process or both. Another cause which may account for thickness decrease is water loss [25] or removal of interlayer water with consequent formation of the compact film. The decrease in the film thickness at higher substrate temperatures may be due to a higher evaporation rate of the initial ingredients of the solution. The thickness values for films at various substrate temperatures are given in Table 1.
A simple reaction mechanism for deposition of copper selenide thin films can be written as follows:
In the present case, copper ions are released into the bath by the dissociation equilibria presented by the complexes of copper:
The selenide ions are released due decomposition of selenourea as,

In presence of metal copper ions the reaction will be,
3.2 Structural analysis
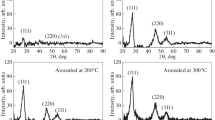
The crystal structure of copper selenide thin films was studied by X-ray diffraction with Cu–Kα radiation (1.5406 Å). The range of 2θ angle was from 10 to 80o. Figure 2 shows typical X-ray diffractogram of copper selenide film deposited at 350 °C. X-ray diffraction patterns reveal that the films of copper selenide deposited by spray pyrolysis technique are polycrystalline in nature. It was observed that the high intensity reflection peaks at 2θ = 31.10º (0 0 6) plane, 2θ = 26.18º (1 0 1) plane, and 2θ = 28.02º (1 0 2) plane 2θ = 66.46º (2 0 7) plane for CuSe thin film. A comparison of observed and the standard ‘d’ values for (h k l) planes ensures that copper selenide shows hexagonal (mineral klockmannite) crystal structure irrespective of substrate temperature [26]. Additionally peaks corresponding to Cu3Se2 (Tetragonal) [27] were also detected with less intensity. Although, there are more than eight stoichiometries of copper selenide, and some stoichiometries have different phases each of them has their own characteristic X-ray diffraction pattern [28]. Therefore, the X ray diffraction patterns give the most positive evidence of the formation of CuSe phase, which was desired. After refinement, the cell constants were calculated to be a = b = 3.969 Å and c = 17.059 Å; which are consistent with the reported data [27].
The formation of Cu3Se2 (Tetragonal) preceding a CuSe deposition can be explained by the fact that copper ions are labile in aqueous media and equilibrium is rapidly established in solutions. The concentration of selenide ions is limited by the selenourea decomposition. The decomposition process takes several minutes to occur. Hence the supersaturation of the solution with respect to Cu3Se2 is established rapidly after the mixing of the reagents, and Cu3Se2 (Tetragonal) may be deposited at the initial stage of the process. Similar results were obtained by Zainal et al. [20]. It is observed that as the substrate temperature increases, the intensity of the peaks also increases and the width of the peak decreases due to improved crystallinity up to 350 °C, for further increase in substrate temperature there is a decrease in peak intensity.
Crystallite size was estimated by using Scherrer’s formula given by the equation,
where k varies from 0.89 to 1.39. But in most of the cases it is closer to 1. Hence for grain size calculation it is taken to be one, λ is wavelength of X-ray, β is the full width at half of the peak maximum in radians and θ is Bragg’s angle. The crystallite size was estimated for the standard (0 0 6) reflection. The crystallite size for copper selenide thin films was found to be in the range of 23–28 nm. The values crystallite sizes are given Table 1.
3.3 Surface morphological and compositional analysis
The surface morphology of copper selenide thin films was investigated using SEM and AFM technique. SEM has been proved to be a unique, convenient and versatile method to analyze surface morphology of thin film and to determine the grain size. Figure 3 shows the SEM micrograph of copper selenide thin films deposited at a substrate temperature of 350 °C and at 10,000× magnifications. From SEM image, it is observed that the as-deposited copper selenide film is uniform, without cracks with dense surface morphology covering the entire substrate surface area. Small nano sized grains were uniformly distributed over smooth homogenous background. The grains are small with uniform and well-defined grain boundaries. The average grain size observed is 85 nm. There is a lack of good agreement between grain sizes calculated from XRD and SEM. This may be due to two or more grains fusing together to form the cluster type of structure.
Figure 4 shows a typical two-dimensional (2D) and three-dimensional (3D) AFM image of the copper selenide thin film deposited at a substrate temperature of 350 °C. The 2D image of the film resembles very well with the morphology of SEM micrograph (Fig. 3). The surface roughness is 1.27 nm. The surface roughness is unavoidable due to three-dimensional growth of the film [29, 30].
The quantitative analysis of the films was carried out by using the EDAX technique for the as-deposited films at different substrate temperatures to study the stoichiometry of the films. Figure 5 shows the typical EDAX pattern for spray deposited copper selenide thin film at a substrate temperature of 350 °C. The elemental analysis was carried out only for Cu and Se; the average atomic percentage of Cu: Se was 52.91:47.09. It is close to 1:1 for as-deposited CuSe film at 350 °C. Similar results for CuSe were reported by Li et al. [31] using EDAX.
3.4 Optical analysis
The optical band gap energy Eg can be ascertained from the experimental values of absorption coefficient α as a function of photon energy hυ, using the following relation
where A is the constant, Eg is the bandgap energy, hυ is the photon energy, n = ½ or 2 for direct or indirect transition. The value of absorption coefficient is found to be of the order of 104 cm−1. The plot of (αhυ)2 versus (hυ) is shown in Fig. 6a which is linear at the absorption edge, indicating a direct allowed transitions. The straight line portion was extrapolated to the energy axis and when (αhυ)2 = 0, the intercept gives the bandgap energy of CuSe. The band gap energy is found to be 2.29 eV for film deposited at 350 °C comparable with values reported earlier [8, 32, 33]. The variation of bandgap energy Eg with the substrate temperature depicted in Fig. 6b. It is found that bandgap energy Eg decreases with a substrate temperature reaches a minimum value 2.29 eV at 350 °C and further increases with increase in substrate temperature. The optical band gap values are found to be in the range 2.29–2.36 eV and are given in Table 1.
3.5 Electrical conductivity analysis
The measurements of electrical conductivity of the CuSe thin film deposited at various substrate temperatures were carried out in the temperature range 300–500 K on rectangular-shaped samples with a typical size of 100 mm2, using a standard DC two point probe method under dark. A plot of inverse absolute temperature versus log (conductivity) for a cooling cycle for copper selenide film deposited at 350 °C is shown in Fig. 7. The conductivity increases with increase in temperature which is the indication of typical semiconductor characteristics. The room temperature electrical conductivities of the copper selenide thin films are in the range between 4.17 × 10−4 and 3.54 × 10−4 (Ω cm)−1. The low value of the room temperature electrical conductivity can be attributed to the dislocations and imperfections of the films. This behavior may also be attributed to the nanocrystalline nature of the thin films, crystallite boundary discontinuities, presence of surface states, thickness of the films, and the change in structural parameter. Likewise, the presence of a number of defects such as structural dislocations, disorders and surface imperfections play an important role in diminishing the conductivity. The conductivity results of the thin films are consistent with other studies [8, 33–35].
In order to calculate the activation energy of the chemically deposited CuSe films, the electrical conductivity results were used. The values of activation energies (Ea) were determined by the Arrhenius equation given by [36]:
where σ and σ0 electrical conductivities, K is the Boltzmann constant and T is the absolute temperature. The Ea is found to be in the range between 0.36 and 0.58 eV in the low temperature region and 0.65–0.86 eV in the high temperature region. The values of the activation energy indicate that the prepared samples are semiconductors. The electrical conductivity results of the CuSe thin films are given in Table 2.
3.6 Hall Effect analysis
From the Hall Effect measurements, the positive polarity of Hall coefficient, it was evident that the as-deposited copper selenide films are p-type semiconductor. The variation of film carrier concentration (n) and room temperature mobility (μ) plotted against substrate temperatures shown in Fig. 8. Carrier concentration increases with a substrate temperature up to 350 °C and decreases thereafter. The increase carrier concentration observed at 350 °C can be related to both the observed improvement in crystallinity of the copper selenide thin films without any residual amorphous content and to a pin hole free surface due to the fact that these films grown at the optimal temperature mediated a CVD process. The reduction in carrier concentration at higher temperature can be related to the decrease in the oxygen vacancies; at higher temperatures, there is a greater incorporation of oxygen to the films. It was found that the variation of mobility is minor compared to that of carrier concentration. It is also worth mentioning that the as-deposited films provided moderate Hall mobility values. It is well known that Hall mobility is related to S (scattering factor) which includes grain boundary related Sg, carrier concentration related Sc and lattice defect related Sd.
This indicates that Hall mobility is determined by a complex of many types of scattering factor [9]. The values of carrier concentration and mobility for the film deposited at 350 °C are 5.02 × 1017 cm−3 and 5.19 × 10−3 cm2 V−1 s−1; respectively with p type conductivity. The values of carrier concentration and mobility for copper selenide thin films deposited at various substrate temperatures are given in Table 2.
3.7 Thermoelectric power (TEP) analysis
The type of conductivity exhibited by the copper selenide thin films is confirmed by TEP measurements. The TEP is defined as the ratio of thermally generated voltage to the temperature difference across a piece of semiconductor. Figure 9 shows the variation of thermo voltage versus temperature difference for as-deposited copper selenide thin film deposited at 350 °C. The polarity of thermo-emf was negative towards the hot end with respect to the cold end, which confirms the fact that CuSe films are of p-type.
4 Conclusions
In summery, copper selenide thin films have been successfully deposited using the simple and inexpensive spray pyrolysis technique. Nearly stoichiometric films with better structural, compositional, optical and electrical properties can be obtained at a substrate temperature of 350 °C. XRD study revealed the polycrystalline nature of the films with hexagonal (mineral klockmannite) crystal structure irrespective to substrate temperatures. The crystallite size is found to be in the range of 23–28 nm. Scanning electron microscopy studies reveal uniform deposition with the average grain size of 85 nm. The optical absorption study revealed direct band gap nature with the bandgap energy in the range 2.29–2.36 eV. Electrical conductivity studies support the fact that the films are semiconductors and can be used in Opto-electronic devices. The values of carrier concentration and mobility for the film deposited at 350 °C are 5.02 × 1017 cm−3 and 5.19 × 10−3 cm2 V−1 s−1; respectively with p-type conductivity.
References
M. Gratzel, Nature 414, 338 (2001)
M. L. Gaur, P. P. Hankare, K. M. Garadkar, S. D. Delekar, V. M. Bhuse, J. Mater. Sci. Mater. Elect. (Article in press 2013). doi:10.1007/s10854-013-1572-9
A.A. Yadav, E.U. Masumdar, Sol. Ener. 84, 1445 (2010)
A.A. Yadav, E.U. Masumdar, J. Alloys Compd. 509, 5394 (2011)
A.A. Yadav, E.U. Masumdar, Electrochim. Acta. 56, 6406 (2011)
M.R. Asabe, V.P. Ubale, A.H. Manikshete, V.T. Vader, S.V. Rajmane, S.D. Delekar, J. Mater. Sci. Mater. Elect. 24, 4655 (2013)
A.A. Yadav, M.A. Barote, E.U. Masumdar, Sol. Ener. 84, 763 (2010)
M.J. Deen, F. Pascal, J. Mater. Sci. Mater. Elect. 17, 549 (2006)
Y.Z. Li, X.D. Gao, C. Yang, F.Q. Huang, J. Alloys Compd. 505, 623 (2010)
A.L.N. Stevels, F. Jellinek, Recueil 111, 273 (1971)
W.S. Chen, J.M. Stewart, R.A. Mickelsen, Appl. Phys. Lett. 46, 1095 (1985)
R.S. Mane, C.D. Lokhande, Mater. Chem. Phys. 65, 1 (2000)
P. Zou, L. Wan, S. Pan, M. Meng, Z. Guo, J. Xu, J. Mater. Sci. Mater. Elect. 24, 4530 (2013)
J.-F. Han, C. Liao, T. Jiang, H.-M. Xie, J. Mater. Sci. Mater. Elect. 24, 4636 (2013)
N.-H. Kim, O. Seongha, W.-S. Lee, J. Korean Phys. Soc. 61, 1177 (2012)
J. Li, H. Kou, Y. Jiang, L. Daban, Z. Zheng, C. Wang, J. Solid State Electrochem. 16, 3097 (2012)
H. Yang, J. Yu, S. Hu, J. Solid State Electrochem. 19, 477 (2009)
J.O. Thompson, M.D. Anderson, T. Ngai, T. Allen, C. David, J. Alloys Compd. 509, 9631 (2011)
J. Ying Chyi Liew, A. Zainal Talib, W. Mahmood, M. Yunus, Z. Zainal, H.A. Shaari, M.M. Moksin, W.M. Yusoff, K.P. Lim, Cent. Eur. J. Phys. 7, 379 (2009)
Z. Zainal, A. Kassim, M.Z. Hussein, C.H. Ching, Mater. Lett. 58, 2199 (2004)
J.M. Villalvilla, J. Gonzalez Velasco, Mater. Chem. Phys. 19, 341 (1988)
Z. Yan, Y. Zhao, M. Zhuang, J. Liu, A. Wei, J. Mater. Sci. Mater. Elect. (Article in press 2013)
M. Kemmler, M. Lazell, P. O’Brien, D.J. Otway, J.-H. Park, J.R. Walsh, J. Mater. Sci. Mater. Electron. 19, 531 (2002)
L.J. Van der Pauw, Philips. Res. Reports. 13, 1 (1958)
A.A. Yadav, E.U. Masumdar, A.V. Moholkar, M. Neumann-Spallart, K.Y. Rajpure, C.H. Bhosale, J. Alloy Compds 488, 350 (2010)
JCPDS Data Card no. 34-0171
JCPDS Data Card no. 72-1421
Powder Diffraction File, Inorganic phases, Alphabetical Index, JCPDS, PA, 1985, p. 264; 671
A.A. Yadav, M.A. Barote, E.U. Masumdar, Mater. Chem. Phys. 121, 53 (2010)
A.A. Yadav, M.A. Barote, E.U. Masumdar, Solid State Sci. 12, 1173 (2010)
H.-L. Li, Y.-C. Zhu, S. Avivi, O. Palchik, J.-P. Xiong, Y. Koltypin, V. Palchik, A. Gedanken, J. Mater. Chem. 12, 3723 (2002)
A.B. Al-Mamun, M.O. Islam, A.H. Bhuiyan, J. Mater. Sci. Mater. Electron. 16, 263 (2005)
A.S. Khomane, Arch. Appl. Sci. Res. 4(4), 1857 (2012)
H.M. Pathan, C.D. Lokhande, D.P. Amalnerkar, T. Seth, Appl. Surf. Sci. 211, 48 (2003)
H. Morikawa, Jpn. J. Appl. Phys. 11, 431 (1972)
K.J. Laidler, The world of physical chemistry (Oxford University Press, Oxford, 1993)
Acknowledgments
Dr. A. A. Yadav is grateful to the SERB, Department of Science and Technology, New Delhi, India for the financial assistance through the Project under SERC Fast Track Scheme for Young Scientist (File No. SB/FTP/PS-068/2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, A.A. Nanocrystalline copper selenide thin films by chemical spray pyrolysis. J Mater Sci: Mater Electron 25, 1251–1257 (2014). https://doi.org/10.1007/s10854-014-1717-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-1717-5