Abstract
CdTe thin film have been deposited onto stainless steel and fluorine doped tin oxide coated glass substrates from aqueous acidic bath using electrodeposition technique. The different preparative parameters, such as deposition time, bath temperature, pH of the bath have been optimized by photoelectrochemical (PEC) technique get good quality photosensitive material. The deposited films are annealed at different temperature in presence of air. Annealing temperature is also optimized by PEC technique. The film annealed at 200 °C showed maximum photosensitivity. Different techniques have been used to characterize the as deposited and also annealed (at 200 °C) CdTe thin film. The X-ray diffraction (XRD) analysis showed the polycrystalline nature and a significant increase in the XRD peak intensities is observed for the CdTe films after annealing. Optical absorption shows the presence of direct transition with band gap energy 1.64 eV and after annealing it decreases to 1.50 eV. Energy dispersive analysis by X-ray study for the as-deposited and annealed films showed nearly stoichiometric compound formation. Scanning electron microscopy reveals that spherically shaped grains are more uniformly distributed over the surface of the substrate for the annealed CdTe film. Photovoltaic output characteristics and spectral response of the annealed film have been carried. The fill factor and power conversion efficiency (η) of the cell are found to be 71 and 3.89 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, thin films have attracted much interest because of their varied applications such as semiconducting devices, photovoltaics, optoelectronic devices, radiation detectors, laser materials, thermoelectric devices, solar energy converters, videocon devices, etc. [1–5].
Abundant literature is available on the preparation and characterization of semiconductor chalcogenide materials [6–10].
CdTe is a promising material because of its high absorption coefficient and the nearly optimum band gap for the efficient absorption of solar radiation. In the conventional solar cells, the wide band gap hetero-junction partner CdS is first deposited on the transparent conducting glass substrate and the absorber layer CdTe is developed over the CdS layer [11–14]. CdTe films grown onto very thin stainless steel, nickel and molybdenum substrates have also been reported [15–17]. The electrical and optoelectronic properties of electrochemically deposited CdTe thin films as well as [18, 19] structural and morphological properties of CdTe have been reported by numbers of investigators [20, 21]. Krishna et al. have studied the effect of electric field on spray deposited CdTe thin films [22]. Photoelectrochemical (PEC) characterization of CdTe in Nafion electrolyte is reported by Krishna et al. [23]. Reports about the effect of the post-deposition heat treatment on the structural changes of CdTe thin films and its influence on the performance of CdTe based solar cells are also available [23, 24].
In the present work, electrochemical technique for depositing CdTe thin films using a single parameter control of deposition potential is reported. The effect of annealing on structural, optical, compositional, microstructural and PEC properties of electrodeposited CdTe thin films has been studied using X-ray diffraction (XRD), optical absorption, energy dispersive analysis by X-ray (EDAX), scanning electron microscopy (SEM) and PEC techniques respectively.
2 Experimental
Preliminary investigations were carried out by cyclic voltammetric studies on deposition of CdTe to establish the conditions for deposition of Cd and Te in aqueous acidic medium. For CdTe deposition studies, solution containing 0.1 M CdSO4 and 150 ppm TeO2 as source for Cd and Te respectively while, stainless steel and fluorine doped tin oxide (FTO) coated glass substrates were used as cathode and graphite plate as a reference electrode. De-ionised water of 16.4 Mohm was used for the preparation of aqueous solution of the above precursor chemicals.
The pH of the electrolytic solution was kept constant by dilute H2SO4. The preparative parameters such as growth time, pH of the bath and bath temperature were optimized by noting short circuit current (Isc) and open circuit voltage (Voc) of the PEC cell formed by the films deposited at various preparative parameters to get more photosensitive CdTe thin films.
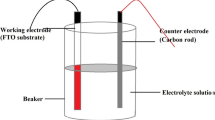
The PEC cell was fabricated by using CdTe thin film as active photoelectrode, polysulphide (0.1 M NaOH + 0.1 M Na2S + 0.1 M S) solution as an electrolyte and graphite as a counter electrode and was illuminated by 200 W tungsten filament lamp. The water compartment was inserted between the cell and lamp to avoid the direct heating of the cell. The films deposited at optimized preparative parameters were annealed at different temperatures. Annealing temperature was also optimized by PEC technique. Experimental set-up for electrodeposition technique is shown in Fig. 1.
The as-deposited and annealed films were used for further characterization by XRD, optical absorption, EDAX, SEM and PEC techniques in order to study the structural, optical, compositional, morphological and PEC properties.
The X-ray diffraction patterns for CdTe thin films deposited onto stainless steel and FTO coated substrates were recorded by Philips X-ray diffractometer model 1710 with Cr-Kα radiation in the span of angle between 10° and 100°.
The optical absorption studies were carried using UV–VIS-IR spectrophotometer Hitachi model 119 in the wavelength range 380–950 nm.
The surface morphology was studied by using JEOL, JXA-840 reflection scanning electron microscopy using magnification of 5,000× at potential 20 kV with EDAX arrangement model.
The fill factor (ff) and power conversion efficiency (η) of the electrodeposited CdTe material were carried out by using above-mentioned PEC cell.
3 Results and discussion
Figure 2 shows the voltammogram of CdTe taken at scan rate of 40 mV/s from 0 to −0.65 V cathodic with reversal to 0 V followed by the anodic sweep up to +0.65 V and then back to zero. During the cathodic scan, a sudden decrease in current occurred in the potential range of −0.30 to −0.45 V and a peak was observed at a potential of about −0.4 V. Since Te is nobler than Cd, the deposition of Te is expected to precede the deposition of Cd. The first cathodic onset therefore indicates the reduction process for Te. On decreasing potential further a plateau region is observed between potential range of −0.4 to 0.6, during which a decrease in potential does not alter current appreciably. A sharp decrease in current on further reduction in potential to about −0.52 V (SCE) corresponds to deposition of CdTe. The anodic back sweep resulted in Cd stripping and initiation of a reaction opposite to Te reduction.
Optimization of preparative parameters for deposition of good quality stoichiometric CdTe thin films is most essential. Optimization of preparative parameters is carried out by noting the maximum values of Isc and Voc of the PEC cell. Figure 3 shows the variation of Isc and Voc with deposition time. From the graph it is observed that Isc and Voc increases with increase in deposition time, attains maximum values for film deposited at 50 min and further increase in deposition time both Isc and Voc decrease this indicates that the formation of good quality and almost stoichiometric compound at 50 min. The lower values of Isc and Voc may be originated due to increase in resistivity of CdTe thin films deviated from stoichiometry [5, 9]. The PEC cell with configuration CdTe/0.1 M polysulphide/graphite is used to check the type of conductivity exhibited by CdTe thin films. The polarity of dark voltage is negative towards CdTe photoelectrode and positive towards the graphite electrode for all samples showing n-type semiconducting behavior. Figure 4 shows the variation of Isc and Voc with bath temperature. From the graph it is observed that both Isc and Voc increase with increase in bath temperature and attain maximum values for the film deposited at 70 °C, indicating probably a better formation of stoichiometric semiconducting compound. Further increase in bath temperature results in decreased values of Isc and Voc. The lower values of Isc and Voc may be attributed to non-stoichiometric growth of CdTe thin films due to insufficient thermal energy provided during the deposition [5]. Figure 5 shows the variation of Isc and Voc with pH of bath, which shows that Isc and Voc are maximum at pH = 3. This indicates that the formation of good quality photovoltaic material is possible from acidic aqueous bath [9]. Electrodeposition of CdTe films was carried out at a potentiostatic mode at −520 mV/SCE. 1–2 μm thick layer was obtained within 50 min of deposition time.
Annealing of the film deposited at optimized preparative parameters (deposition time = 50 min, bath temperature = 70 °C and pH = 3) was carried out in air at four different temperatures 100, 150, 200, 250 °C for 60 min. Figure 6 shows the variation in Isc and Voc with annealing temperature, which shows that Isc and Voc are maximum for the annealing temperature of 200 °C. It seems that heating at 200 °C in air produces more realignment in orientation leading to improved crystallinity and stoichiometry. Further increase in annealing temperature above 200 °C, Isc and Voc decrease this may be due to peeling off material from surface of substrate resulting in deviation from stoichiometry in CdTe thin film. After annealing the CdTe samples showing p-type semiconducting behavior.
The XRD pattern shows the sharp peak and reveals the polycrystalline nature of the as deposited CdTe films. The structural features fit into cubic one. The XRD patterns for the as prepared and the annealed films at 200 °C obtained are shown in Fig. 7a, b respectively. A significant increase in the XRD peak intensities is observed for the CdTe film annealed at 200 °C as compared to the peaks of as deposited sample. This reveals that the as deposited films were well crystallized and heating in air produces more realignment in orientation leading to improved crystallinity. Thus, the annealed thin films seem to be more suitable for device applications. The optical absorption of the as-deposited and annealed CdTe thin films has been studied in the range 380–950 nm. The variation of optical density with wavelength was analyzed to find out the nature of transition involved and the optical bandgap, using the relation [9].
where the symbols have their usual meanings.
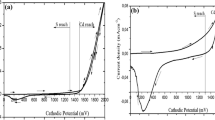
For allowed direct transition, n = ½, the value of absorption coefficient is of the order of 104 cm−1 supporting the direct band gap nature of the semiconductor. The plot of (αhυ)2 versus hυ for typical sample deposited at optimized preparative parameters (deposition time 50 min, bath temperature 70 °C and at pH 3) and annealed at 200 °C are shown in Fig. 8a, b. The linear nature of the plot indicates the presence of direct transition. The linear portions are extrapolated to energy axis at α = 0, giving the band gap energies of as-deposited and annealed CdTe films to be 1.64 and 1.50 eV respectively. Decrease in band gap after annealing may be due to more realignment in orientation leading to improved crystallinity and stoichiometry.
The compositional analysis for the as-deposited and annealed CdTe films are carried out by EDAX technique. The compositions for as-deposited and annealed (200 °C) CdTe thin films are tabulated in Table 1. EDAX study shows that the Cd to Te ratio is ~1 for as-deposited as well as annealed CdTe thin films.
The surface morphology of CdTe thin film was studied by SEM picture. Figure 9a, b shows a surface morphology of the as deposited and annealed film (200 °C) exhibiting its microstructure. The film after annealing shows smooth and uniform surface with a crack free appearance with spherical shaped grains.
The photoresponse of the PEC cell is measured by noting Isc and Voc as a function of light intensity f L. The equivalent diagram of PEC cell implies that Isc varies linearly with the light intensity as [24],
where C is a constant. Variation of short circuit current I sc and open circuit voltage V oc as a function of light intensity for the PEC cell is depicted in Fig. 10. It is seen that I sc varies linearly over the whole range of light intensities under study. However, at higher intensities saturation in V oc is observed which can be attributed to the saturation of the electrolyte interface charge transfer and nonequilibrium distribution of electrons and holes in the space charge region of the photoelectrode, which contribute a great deal to open cell photovoltage [25]. In case of solid junction, this deviation is attributed to the series resistance of the cell.
The relation between Voc and light intensity for equilibrium distribution of the charge carriers in the space charge region is given by [26],
From Fig. 9, it is seen that the Voc saturates at higher intensities, which is good agreement with the theory.
The plot of Isc against wavelength is shown in Fig. 11. Isc attains maximum value at wavelength 819 nm and decreases with further increase of wavelength. The decrease of Isc on shorter wavelength side may be due to the absorption of light in the electrolyte and high surface recombination of photogenerated carriers by the surface states. The decrease in Isc on the longer wavelength side may be attributed to the non-optimized thickness and transition between defect levels. The maximum Isc is obtained corresponding to wavelength 819 nm which gives the band gap 1.50 eV, agreeing with the result of optical absorption studies.
Figure 12 shows the photovoltaic power output characteristics for a typical cell (CdTe/0.1 M NaOH + 0.1 M Na2S + 0.1 M S/C) under the illumination intensity of 30 mW/cm2. The short circuit current Isc and open circuit voltage Voc are found to be 3.2 mA and 598 mV respectively. The ff and power conversion efficiency (η) of the cell are 71 and 3.89 % respectively.
4 Conclusion
An electrochemical route for the preparation of CdTe thin films and effect of post annealing is studied. In order to prepare good quality deposits optimization of preparative parameters by PEC technique is suitable. The optical, compositional and morphological, analysis of as-deposited as well as annealed films has been carried out. The energy gap of the material is direct type with band gap energy (Eg) 1.64 eV and after annealing it decreases to 1.50 eV. Films prepared using the optimized deposition parameters show preferential orientation along (111) plane. Annealing treatment of the film in air shows an improvement in the polycrystalline nature of the film. From EDAX result Cd to Te ratio is almost found to be ~1. The SEM study shows that smooth and uniform growth of spherical shaped grains on surface substrate with a crack free appearance. The ff and power conversion efficiency (η) of the cell are 71 and 3.89 % respectively.
References
X. Mathew, P.J. Sebastian, Sol. Energy Mater. Sol. Cells 59, 85 (1999)
H.J. Goldsmid, J.E. Giutronich, M.M. Kalia, Sol. Energy 24, 435 (1980)
N.G. Patel, P.G. Patel, Solid State Electrochem. 35, 1269 (1992)
R. Chandramohan, T. Mahalingam, J.P. Chu, P.J. Sebastian, Sol. Energy Mater. Sol. Cells 81, 371 (2004)
S.K. Deshmukh, A.V. Kokate, D.J. Sathe, Mater. Sci. Eng. B 122, 206 (2005)
S.K. Pandey, U. Tiwari, R. Raman, C. Prakash, V. Krishna, V. Dutta, K. Zimik, Thin Solid Films 473, 54 (2005)
B.E. McCandless, K.D. Dobson, Sol. Energy 77, 839 (2004)
X. Mathew, J. Phys. D Appl. Phys. 33, 1565 (2000)
A.V. Kokate, U.B. Suryavanshi, C.H. Bhosale, J. Sol. Energy 80, 156 (2006)
P.P. Hankare, S.D. Delekar, V.M. Bhuse, P.A. Chate, K.M. Garadkar, Semicond. Sci. Technol. 20, 257 (2005)
Y. Wada, S. Nishimatsu, J. Electrochem. Soc. 125, 1499 (1978)
A. Morales-Acevedo, Sol. Energy Mater. Sol. Cells 90(6), 678 (2006)
J. Van Gheluwe, J. Versluys, D. Poelman, P. Clauws, Thin Solid Films 480, 264 (2005)
J. Terrazas, A. Rodríguez, C. Lopez, A. Escobedo, F.J. Kuhlmann, J. McClure, D. Zubía, Thin Solid Films 490, 146 (2005)
S.S. Hegedus, B.E. McCandless, Sol. Energy Mater. Sol. Cells 88, 75 (2005)
A.E. Rakhshani, J. Appl. Phys. 81, 7988 (1997)
V.P. Singh, J.C. McClure, G.B. Lush, W. Wang, X. Wang, G.W. Thompson, E. Clark, Sol. Energy Mater. Sol. Cells 59, 59 (1999)
S.A. Gamboa, P.J. Sebastian, M.A. Rivera, Sol. Energy Mater. Sol. Cells 52, 293 (1998)
A.E. Rakhshani, Y. Makdisi, X. Mathew, N.R. Mathew, Phys. Stat. Sol. A 168, 177–187 (1998)
X. Mathew, J. Appl. Phys. 33, 1565 (2000)
S. Lalitha, R. Sathyamoorthy, S. Senthilarasu, A. Subbarayan, K. Natarajan, Sol. Energy Mater. Sol. Cells 82, 187 (2004)
J.P. Enríquez, X. Mathew, Sol. Energy Mater. Sol. Cells 81, 363 (2004)
K. Vamsi Krishna, V. Dutta, P.D. Paulson, Thin Solid Films 444, 17 (2003)
A. Romeo, D.L. Batzner, H. Zogg, A.N. Tiwari, Thin Solid Films 361, 420 (2000)
J.D. Desai, C.D. Lokhande, Mater. Chem. Phys. 34, 313 (1993)
V.V. Killedar, C.D. Lokhande, C.H. Bhosale, Indian J. Pure Appl. Phys. 36, 643 (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asabe, M.R., Ubale, V.P., Manikshete, A.H. et al. Properties of electrochemically deposited CdTe thin films: annealing effect. J Mater Sci: Mater Electron 24, 4655–4661 (2013). https://doi.org/10.1007/s10854-013-1457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-013-1457-y