Abstract
In this study, YPO4:Eu3+ microspheres with different Eu3+ dosage concentration were fabricated by a facile hydrothermal route at 200 °C for 10 h in the presence of citric acid. The YPO4:Eu3+ samples were characterized by X-ray powder diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and luminescence spectroscopy. The XRD results reveal that the YPO4:Eu3+ samples presented a tetragonal structure. The TEM and SEM observations demonstrate that the YPO4:Eu3+ samples with uniform sphere-like morphologies can be obtained at 200 °C for 10 h. The sizes of samples are in the range of 2–2.2 μm. The room temperature luminescence properties of YPO4:Eu3+ samples were studied using an excitation wavelength of 227 nm. The emission spectrum displays the bands associated to the 5D0 → 7FJ (J = 1, 2 and 4) electronic transitions characteristics of the Eu3+ cations at different positions. The influence of Eu3+ dosage concentration on luminescence properties of YPO4:Eu3+ microspheres were studied carefully.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, more and more attentions have been focused on the study of the rare earth ions doped inorganic materials for application in luminescence materials [1]. The rare earth ions doped materials are the best phosphors for the generation of red, green, and blue colors in various applications [2]. Also, rare earth ions doped phosphors have some advantages when compared with other available luminescence materials (quantum dots or organic dyes), such as low toxicity, photostability, high thermal and chemical stability, high luminescence quantum yield (QY), and sharp emission bands [3]. Meanwhile, the luminescence properties can be tuned easily by adjusting the concentration of the rare earth ions [4]. Generally speaking, rare-earth doped orthophosphates, RPO4 (R: Y, La, Gd, and Bi), have absorption edges at rather short wavelengths so that they are suitable for hosts of phosphors transparent in the wavelength region of the vacuum ultraviolet (VUV) [5–7]. For example, YPO4 exhibits high thermal and chemical stability because Y3+ has an empty 4f electron shell with a stable structure [8]. It had been reported that YPO4 is a good luminescence host for rare earth ions [9]. Furthermore, the YPO4:Eu3+ phosphors are found to be one of the most familiar red emitting phosphors, and have been explored successfully for the design of luminescence devices [10].
It is well known that the intrinsic properties of inorganic materials are determined by their sizes, shapes, morphologies, compositions, and crystallinity [11]. The phosphor particles with spherical shape can offer the possibility of brighter cathodoluminescent performance, high definition, much improved screen packing and low scattering of light [12, 13]. In addition, spherical phosphor particles are able to increase the screen brightness and improve the resolution [14]. Therefore, YPO4:Eu3+ phosphors with spherical morphology were synthesized by different methods [15, 16]. But there are hardly any reports about the influence of the Eu3+ dosage concentration on luminescence properties of YPO4:Eu3+ microspheres. In the present work, Eu3+-doped YPO4 were synthesized by a surfactant-assisted approach. Especially, the influence of the Eu3+ dosage concentration on luminescence properties of YPO4:Eu3+ microsphere was studied carefully.
2 Experimental
All the chemical reagents were of analytical grade and used as received without further purification. All chemical reagents were obtained from Nanjing Chemical Reagent Co., Ltd, China. Experimental details include following steps. Firstly, 2.5 mmol of Ln(NO3)3 (Ln = Y and Eu, with different mole ratios of Y:Eu) was dissolved in 15 ml of deionized water (Table 1), and then 0.10 g of citric acid (C6H8O7) was introduced into the solution. Secondly, 2.5 mmol of (NH4)2HPO4 was dissolved in 15 mL of deionized water, too. Thirdly, the (NH4)2HPO4 solution was added drop-wise into the Ln(NO3)3 solution under vigorous stirring, until a white-colored solution was obtained. Finally, the resulting suspension was transferred into a 40 ml Teflon-lined stainless steel autoclave with a filling capacity of 75 % and maintained at 200 °C for 10 h. After the hydrothermal system was cooled to room temperature naturally, the precipitates were collected, washed several times with distilled water and dried in air at 80 °C for 8 h.
The X-ray diffraction (XRD) patterns of the samples were examined using a Japan RigaKu D/max-g X-ray diffractometer system with graphite monochromatized Cu Kα irradiation (λ = 1.5418 Å). The transmission electron microscopy (TEM) was taken using a JEM-100CX TEM running at an accelerating voltage of 100 kV. The scanning electron microscopy (SEM) images were taken on a JEOL JSM-6700F scanning electron microscope. The excitation and emission spectra of the samples were measured by an Edinburgh FLS920 fluorescence-lifetime and steady-state spectrometer. All the measurements were taken at room temperature.
3 Results and discussions
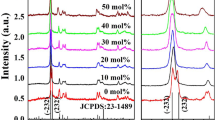
Figure 1 shows the XRD patterns of the as-obtained Eu0.05Y0.95PO4 samples. It reveals that all the diffraction peaks can be indexed to tetragonal structured YPO4 readily according to JCPDS-840335. No obvious impurity phase is found in the samples. The XRD patterns are similar for YPO4:Eu3+ samples with different Eu3+ dosage concentrations.
The SEM and TEM images of the Eu0.05Y0.95PO4 samples are shown in Fig. 2. From Fig. 2, one can see that the formation of a large quantity of spherical particles in micrometer scale. The sizes of Eu0.05Y0.95PO4 microspheres are in the range of 2–2.2 μm. A SEM micrograph of Eu0.05Y0.95PO4 particles shown in the inset of Fig. 2 reveals that spherical particles have smooth surface. The morphologies are similar for YPO4:Eu3+ samples with different Eu3+ dosage concentrations.
Figure 3 displays the excitation and emission spectra of the Eu0.05Y0.95PO4 microspheres. The excitation spectrum consists of a strong band centered at 227 nm and a band centered at 397 nm. The strong band centered at 227 nm is due to the oxygen-to-europium charge transfer band (CTB) [17]. The band centered at 397 nm is attributed to the 7F0 → 5L6 transition of the Eu3+ ions. The emission spectrum is monitored at the most intense Eu3+ emission wavelength. As observed, it consists of several sharp lines due to the direct emission of the Eu3+ cations from the ground state to higher levels of the 4f-manifold. The emission spectrum displays the bands associated to the 5D0 → 7FJ (J = 1, 2 and 4) electronic transitions characteristics of the Eu3+ cations at different positions. Among them, the 5D0 → 7F1 transitions are the strongest and characterized by an orange–red emission. The 5D0 → 7F1 lines originate from the magnetic dipole transition, while the 5D0 → 7F2 lines originate from the electric dipole transition [18]. The electric dipole transition is allowed only on the condition that the Eu3+ ion occupies a site without an inversion center and is sensitive to local symmetry, which induces the relatively strong of 5D0 → 7F1 transition and the relatively weak of 5D0 → 7F2 transition. In the emission spectrum of the Eu0.05Y0.95PO4 microspheres, the most peak centered at 592 nm (5D0 → 7F1), which is stronger than that of the 618 nm (5D0 → 7F2), suggesting a higher occupancy of Eu3+ in the asymmetric environment. The excitation and emission spectra are similar for YPO4:Eu3+ samples with different Eu3+ dosage concentrations, besides the different emission intensities.
Figure 4 shows the Eu3+ emission intensities of (5D0 → 7F1) transition and QY values for all of samples as a function of Eu3+ dosage concentration. It can be seen that the emission intensities of samples increase with the increases of Eu3+ dosage concentration and reach a maximum value around 5 mol % Eu3+. Firstly, increases of Eu3+ dosage concentration decrease the degree of symmetry in the samples because of the bigger radius of Eu3+. Lower symmetry will lead to higher emission intensity. Secondly, the distance of Eu3+ decreases with the increases of Eu3+ dosage concentration, which enhances the interaction of Eu3+. The decreases of the distance between the luminescence centers will cause the probability of nonradiative energy migration between Eu3+ ions and lead to nonradiative deexcitation, which is known as the concentration quenching. The experimental Eu3+ dosage concentration in YPO4 of concentration quenching is 5 mol %. The QY value also continues to increase with the increase of Eu3+ concentration up to 5 mol %, where a maximum QY value of 0.49 is acquired. This is in good agreement with the tendency observed in emission intensity.
The decay process of YPO4:Eu3+ phosphors with different Eu3+ dosage concentration are investigated and shown in Fig. 5. All the curves can be well fit into a single-exponential function as I(t) = I0 exp(−t/τ1) (I0 is the initial emission intensity at t = 0 and τ is the 1/e lifetime of the emission center). The average lifetime values are calculated from the decay curves and shown in Fig. 5, too. The lifetime value is found to be maximal (3.15 ms) for Eu0.05Y0.95PO4 sample, which is in accordance with the emission intensities corresponding to the samples shown in Fig. 4. This phenomenon is owing to the fact that the material with long lifetime of the emission transitions would create strong population inversion upon excitation, and hence it would cause high excited state electrons and thereby enhancing the rate of recombination. Therefore, it produces strong fluorescence [19]. With an increase in Eu3+ dosage concentration, the extent of energy transfer from host to Eu3+ increases as there are a number of Eu3+ ions in the lattice to receive energy from the host. This effect will lead to an increase in emission intensities from Eu3+ ions. Hence, the lifetime values of the samples increase with the increases of Eu3+ dosage concentration up to 5 mol %, because of the domination of the host to Eu3+ energy transfer effect. However, there are interactions among Eu3+ ions. The extent of Eu3+–Eu3+ interaction increases due to the reduced Eu3+–Eu3+ internuclear distances and this leads to energy transfer among different Eu3+ ion followed by nonradiative decay of the excited state. Such interactions result in a decrease in the excited state lifetime corresponding to Eu3+ ions. Therefore, the lifetime values of the samples decrease when the Eu3+ dosage concentration is more than 5 mol %, because of the domination of the Eu3+–Eu3+ interaction. In other words, the lifetime is determined by two competing and opposite effects, namely an increase in Eu3+–Eu3+ interaction and an increase in the extent of energy transfer from host to Eu3+ ions.
4 Conclusions
YPO4:Eu3+ microspheres with different Eu3+ dosage concentration were synthesized at temperature of 200 °C. The size of microspheres is in the range of 2–2.2 μm. The emission spectrum of samples displays the bands associated to the 5D0 → 7FJ (J = 1, 2 and 4) electronic transitions characteristics of the Eu3+ cations at a position. It is found that the concentration has an obvious influence on emission intensities, QYs and lifetimes of samples. The emission intensity and the lifetime are determined by two competing and opposite effects, namely an increase in Eu3+–Eu3+ interaction and an increase in the extent of energy transfer from host to Eu3+ ions. The Eu0.05Y0.95PO4 sample has the highest value of emission intensity, quantum yield and lifetime.
References
Y. Zhang, H. He, X. Yang, A. Zheng, Y. Fan, Powder Technol. 224, 175 (2012)
G. Blass, B.C. Grabmaier, Luminescent materials (Springer, Berlin, 1994)
S. Rodriguez-Liviano, F.J. Aparicio, T.C. Rojas, A.B. Hungría, L.E. Chinchilla, M. Ocana, Cryst. Growth Des. 12, 635 (2012)
U. Rambabu, D.P. Amalnerkar, B.B. Kale, S. Buddhudu, Mater. Res. Bull. 35, 929 (2000)
K. Park, M.H. Heo, K.Y. Kim, S.J. Dhoble, Y. Kim, J.Y. Kim, Powder Technol. 237, 102 (2013)
S. Lin, X. Dong, R. Jia, Y. Yuan, J. Mater. Sci.: Mater. Electron. 21, 38 (2010)
S. Liu, W. Zhang, Z. Hu, Z. Feng, X. Sheng, Y. Liang, J. Mater. Sci.: Mater. Electron. 24, 4253 (2013)
H. Lai, A. Bao, Y.M. Yang, Y.C. Tao, H. Yang, Y. Zhang, L.L. Han, J. Phys. Chem. C 112, 282 (2008)
E. Nakazawa, Chem. Phys. Lett. 56, 161 (1978)
O. Guillot-Noël, B. Viana, B. Bellamy, D. Gourier, G.B. Zogo-Mboulou, S. Jandl, Opt. Mater. 13, 427 (2000)
S. Mann, G.A. Ozin, Nature 382, 313 (1996)
M.I. Martinez-Rubio, T.G. Ireland, G.R. Fern, J. Silver, M.J. Snowden, Langmuir 17, 7145 (2001)
G. Wakefield, E. Holland, P.J. Dobson, J.L. Hutchison, Adv. Mater. 13, 1557 (2001)
H. Wang, C.K. Lin, X.M. Liu, J. Lin, M. Yu, Appl. Phys. Lett. 87, 181907 (2005)
M. Yang, H. You, N. Guo, Y. Huang, Y. Zheng, H. Zhang, Cryst. Eng. Comm. 12, 4141 (2010)
L. Zhang, G. Jia, H. You, K. Liu, M. Yang, Y. Song, Y. Zheng, Y. Huang, N. Guo, H. Zhang, Inorg. Chem. 49, 3305 (2010)
L. Yu, H. Song, S. Lu, Z. Liu, L. Yang, X. Kong, J. Phys. Chem. B 108, 16697 (2004)
B.R. Judd, Phys. Rev. 127, 750 (1962)
S. Buddhudu, C.H. Kam, S.L. Ng, Y.L. Lam, B.S. Ooi, Y. Zhou, K.S. Wong, Mater. Sci. Eng., B 72, 27 (2000)
Acknowledgments
This work is supported financially by the Quzhou Municipal Science and Technology Project (Grant No. 2013Y018), and the Natural Science Foundation of Zhejiang Province (Grant No. Y1110557).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Yu, H. Influence of the Eu3+ dosage concentration on luminescence properties of YPO4:Eu3+ microspheres. J Mater Sci: Mater Electron 25, 1013–1016 (2014). https://doi.org/10.1007/s10854-013-1680-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-013-1680-6