Abstract
Polycrystalline cadmium selenide (CdSe) thin films have been synthesized at room temperature by using chemical bath deposition method. The synthesized films were characterized by using X-ray diffraction (XRD), optical absorbance, electrical conductivity, scanning electron microscope, energy dispersive X-ray analysis, photoluminescence and photoelectrochemical (PEC) techniques. The film of 0.84 μm thickness, deposited on glass substrate showed uniform spherical morphology with an optical band gap of 1.99 eV. The XRD analysis confirmed presence of cubic structure. Scanning electron micrograph shows a typical spherical ball like morphology with large surface area, which is useful for absorption of large solar radiation. The conductivity measurements showed n type semiconducting nature of the film. A PEC cell device fabricated using ‘as deposited’ CdSe film as anode showed a stable conversion efficiency of 0.7 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The studies on polycrystalline, semiconducting, binary metal chalcogenide materials belonging to II–VI groups has been a rapidly growing area of research due to their ability to convert solar energy into electricity. The cadmium selenide (CdSe) is one of the promising semiconducting material because of its characteristic size-dependent properties, which allow tailoring of the energy band structure and optoelectronic properties [1–3]. CdSe is of special interest because of its direct optical band gap (1.74 eV). A variety of physical and chemical techniques are available for the deposition of CdSe thin films [4, 5].
A chemical bath deposition (CBD) technique has been used for many decades due to its good applicability for producing large-area thin films. A good adherent thin film can be deposited on any substrate by optimizing the various parameters. The characteristics of deposited film are ‘recipe oriented’ therefore, a large number of studies on this technique have been observed. The recipe oriented characteristics motivate the studies on applications of cadmium selenide films in diversified fields. The semiconducting crystalline thin films find very promising applications in optoelectronics, photonics, transistors, photovoltaic cells and light emitting diodes [6–12].
The metal chalcogenide thin films can be prepared by using various physical and chemical techniques such as solvothermal [1], spray pyrolysis [13], vacuum evaporation [14], sputtering [15], electron beam evaporation [16], electro-deposition [17], photoelectrochemical (PEC) [18], successive ionic layer adsorption and reaction (SILAR) [19], photochemical deposition [20] and CBD [21, 22]. CBD is simple and economically viable technique for producing good quality films with better stiochiometry, suitable for device application.
In the present work, an attempt is made to synthesize polycrystalline CdSe thin films chemically. The films were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), energy dispersive X-ray analysis (EDS), optical measurement techniques, photoluminescence (PL) and PEC studies.
2 Experimental details
2.1 Preparation of substrate and reagents
For synthesis of cadmium selenide thin film, the chemicals used were all of analytical grade (AR). It includes cadmium nitrate [Cd(NO3)2·4H2O], liquor ammonia (2.8 M liq. NH3), sodium sulfite (Na2SO3) and selenium powder (Thomas Baker). All the solutions were prepared in double distilled water.
Sodium selenosulphate (Na2SeSO3) solution was prepared by refluxing 5 g selenium powder and 15 g sodium sulfite in 200 mL double distilled water for 9 h at 363 K [23]. The commercially available, non-conducting micro glass slides (Blue Star, Mumbai) having dimensions of 75 × 25 × 1.35 mm were used for deposition of CdSe films. The slides were cleaned by washing with chromic acid followed by rinsing in acetone and finally with double distilled water before the deposition of film.
For the deposition of CdSe thin film, cadmium nitrate and sodium selenosulphate solution were used as a source of Cd+2 and Se−2 ions respectively while, liquor ammonia was used as complexing agent. The Cd(NH3) 2+4 complex and sodium selenosulphate releases Cd+2 ions and Se2− ions very slowly, which combines on substrate to give uniform CdSe thin film.
2.2 Deposition of cadmium selenide thin film
For deposition of CdSe films, a chemical bath was prepared by mixing 10 mL (0.25 M) cadmium nitrate, 40 mL (2.8 M) liquor ammonia and 15 ml of 30 % triethanolamine (TEA) in a 250 ml beaker. To this 10 mL (0.25 M) sodium selenosulphate was added at room temperature and the final volume was made to 180 ml by adding double distilled water. The pH of the reactive mixture was maintained at 10.5 ± 0.5. The beaker was then kept in oil bath. The cleaned glass slides, mounted vertically on a specially designed substrate holder were rotated at a speed of 35 ± 2 rpm in the reaction mixture. The temperature of the bath was then slowly increased up to 338 K. After about 180 min, the slides were taken out, washed several times with double distilled water, dried naturally and preserved in a dark desiccator over anhydrous CaCl2 and subjected to various characterization.
2.3 Characterization of thin films
The thickness of cadmium selenide thin film was measured by using a weight difference method. The XRD study was carried out with CuKα1 radiation using Philips PW-1710 diffractometer having the wavelength 1.54056 Å. The ‘dc’ electrical conductivity measurements were performed using four probe technique using a quick air drying silver paste. For conductivity measurements, a constant 2 V dc voltage was applied across the sample. In thermoelectric power measurements, the potential difference between the points was recorded by using a digital microvoltmeter. A calibrated thermocouple probe (Chromel–Alumel, 24 gauge) with a digital indicator was used to sense the working temperature. The optical absorption measurements were carried out by using UV (3600 Shimadzu UV–VIS–NIR double beam) spectrophotometer at room temperature in wavelength rang of 400–900 nm. The PL spectrum was recorded on Spectroflurometer (Jasco Spectroflurometer FP—8300 Japan). A SEM and EDS (JEOL-2300) were used for microscopic observations and compositional analyses respectively. The PEC properties were measured on Keithley Model-4200 (SCS).
3 Results and discussion
3.1 Growth mechanism
The deposition of CdSe thin film takes place by temperature triggered decomposition of cadmium complex and sodium selenosulphate solution in basic medium. A slow increase in temperature allow the decomposition of sodium selenosulphate and cadmium–ammonium complex in supporting alkaline medium generating Se−2 and Cd+2 ions, which combines on substrate giving thin film. The deposition take place when the ionic product of Cd+2 and Se−2 exceeds the solubility product [Ksp = 10−33]. The growth of film can be understood from the following reactions;
Initially the growth is fast due to higher concentrations of ions, thereafter it slows down and eventually stops as the ions are depleted. After carrying several trial experiments, the various preparational parameters such as pH of the bath, temperature, concentration of the solution, speed of rotation have been optimized to get good quality films.
3.2 X-ray diffraction analysis
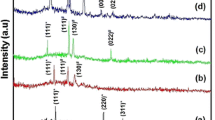
The XRD technique has been used to study crystal structure of the films. The XRD pattern of as-deposited and annealed CdSe films are shown in Fig. 1. The XRD pattern of as deposited and annealed CdSe sample showed prominent peaks at 2θ angles 26.33°, 43.25° and 49.73° corresponding to (111), (220) and (311) orientations, respectively as per JCPDS card number 19-0191, indicating presence of cubic crystal structure. The lattice parameter of the film has been calculated as 6.06 Å, which is in good agreement with the JCPDS card value. The X-ray pattern of CdSe films have a broad base due to amorphous glass substrate. A considerable improvement in peak intensity is observed after annealing at 473 K temperature due to CdSe grain growth.
3.3 Scanning electron microscopy
The SEM is useful technique for determination of surface morphology. The scanning electron micrographs of the ‘as deposited’ cadmium selenide thin film at different magnifications (3,000× and 10,000×) are shown in the Fig. 2a, b. The SEM image show the presence of CdSe grains having excellent spherical ball like morphology. The spherical balls have nearly identical diameters of ~450 nm and are homogenously arranged without interfusion. This type of typical morphology has large surface area, which is useful for absorption of maximum solar radiation.
3.4 Energy dispersive X-ray analysis
The elemental composition of CdSe thin film has been determined from EDS. The EDS spectrum (Fig. 3) show the presence of Cd and Se in 47.52 and 52.48 atomic mass percentages respectively indicating the presence of fair stiochiometry.
3.5 Optical properties
The optical absorption spectrum of CdSe thin film deposited on glass substrate was recorded on the double beam spectrophotometer in the wavelength range of 400–900 nm at room temperature. Figure 4 shows the optical absorption spectrum of CdSe thin film. A considerable decrease in absorption for wavelengths from 525 to 600 nm was observed. The optical absorption coefficient was calculated by using Lambert law [24].
Where, I0 and I are the intensities of incident and transmitted light, respectively; α is the absorption coefficient, A is the optical absorption and d is the thickness of film.
The relationship between absorption coefficient (α) and photon energy (hν) for various types of transitions near the absorption edge is governed by relation [25];
where, B is edge width parameter, x is a factor that determines the nature of transitions involved (for direct allowed transitions, x = ½). In order to find nature of transition involved, Eq. (4) can be rearranged to a line equation, y = mx + c. The obtained data is checked for various values of ‘x’ to obtain a linear fit. Accordingly we obtain a linear fit for x = ½, indicating the presence of a single direct optical transition. Figure 5 shows a liner fit of (αhν)2 versus hν plot for x = ½. The variation is linear beyond band gap. The linear portion is extrapolated to x axis to give optical band (Eg) of magnitude 1.99 eV. The value is in good agreement with the literature reported value (1.92–2.1 eV) [1, 17, 26].
3.6 Electrical properties
The electrical conductivity of cadmium selenide thin film was measured in the temperature range of 300–500 K. An increase in conductivity was observed with increase in temperature confirming the semiconducting nature of the film. The structural characteristics, nature of impurity, type of conductivity etc. decides the important electronic properties of thin film material [27]. The type of conductivity can be determined from the temperature dependence of the electrical conductivity of the material which follows Arrhenius relation;
where, Ea is the activation energy. The other terms have their usual meaning. The variation of log σ with inverse absolute temperature is shown in Fig. 6. The linear nature of graph indicates the presence of single type of conduction mechanism. The activation energy of CdSe film, calculated from the slope of above plot was found to be 0.445 eV. The thermoelectric emf generated when a temperature gradient was applied across the length of CdSe film showed negative polarity toward cold end indicating the presence of n-type conduction carriers.
3.7 Photoluminescence (PL) studies
The PL spectroscopy has received increasing interest due to its nondestructive nature. The PL studies are used to characterize the quality of semiconductor material. It furnish information about band edge emission and deeper defect related emissions often useful to characterize the homogeneity, stiochiometry, presence of any defects along with its nature and doping level etc. [28]. Figure 7 shows PL spectrum of as-deposited CdSe thin film at an excitation wavelength of 510 nm. The spectrum show one strong emission peak at 625 nm (1.98 eV) and other weak emission peak at 680 nm (1.82 eV) wavelength. The strong emission at 625 nm (1.98 eV) corresponds to excitonic band to band (e–h) recombination (direct transition), the obtained value is close to band gap of polycrystalline CdSe thin film (i.e. 1.99 eV). A weaker emission peak at 680 nm (1.82 eV) is attributed to the presence of shallow trap centers. Besides, we observed no other emission peaks confirming the excellent quality of CdSe film.
3.8 Photoelectrochemical properties
The cadmium selenide film is used as a photoanode in a PEC cell. The electrolyte used was 0.25 M polysulfide solution. Figure 8 shows variations of current density against voltage (I–V characteristics) of a PEC device under zero illumination (black) and under a standard illumination of 30 mW/cm2 (red) at room temperature. The photocurrent was found to increase with gradual increase of positive potential, confirming that the CdSe thin film is n-type. The power output of the PEC cell was measured at 30 mW/cm2. The value of short circuit current (Isc) and open circuit voltage (Voc) was found to be 45.5 μA/cm2 and 100 mV respectively under illumination intensity of 30 mW/cm2. The analysis of data showed a power conversion efficiency of 0.70 % and a fill factor of 20.97 % [29–32].
4 Conclusion
The CdSe films, obtained by using chemical deposition technique showed good homogenous nature with identical, spherical ball-like morphology. The XRD study indicated presence of cubic phase, while optical studies revealed a direct band gap of 1.99 eV magnitude. The electrical activation energy was found to be 0.445 eV. A PEC device fabricated using polysulfide as electrolyte showed a light to electrical conversion efficiency of 0.7 % with a fill factor of 20.97 %. The performance of cadmium selenide observed in our work can motivate further studies with respect to solar conversion.
References
T.S. Shyju, S. Anandhi, R. Indirajith, R. Gopalakrishnan, J. Cryst. Growth 337, 38 (2011)
J.B. Chaudhari, N.G. Deshpande, Y.G. Gudage, A. Ghosh, V.B. Huse, R. Sharma Appl, Surf. Sci. 254, 6810 (2008)
R. Henriquez, A. Badan, P. Grez, E. Munoz, J. Vera, E.A. Dalchiele, R.E. Marotti, H. Gomez, Electrochim. Acta 56, 4895 (2011)
K.R. Murali, K. Sivaramamoorthy, M. Kottaisamy, S. Asath Bahadur, Phys. B 404, 2449 (2009)
S.A. Pawar, R.S. Devan, D.S. Patil, A.V. Moholkar, M.G. Gang, Y.R. Ma, J.H. Kim, P.S. Patil, Electrochim. Acta 98, 244 (2013)
Y. Akaltun, M.A. Yildirim, A. Ateş, M. Yildirim, Opt. Commun. 284, 2307 (2011)
A.S. Khomane, Optik 124, 2432 (2013)
T.M. Razykov, C.S. Ferekides, D. Morel, E. Stefanakos, H.S. Ullal, H.M. Upadhyaya, Sol. Energy 85(8), 1580 (2011)
R. Mariappan, V. Ponnuswamy, M. Ragavendar, Optik 123, 1196 (2012)
K.K. Kasem, Mater. Sci. Eng. B 65, 127 (1999)
C. Ma, Z.L. Wang, Adv. Mater. 17, 2635 (2005)
V.M. Bhuse, P.P. Hankare, Ionics 6, 304 (2000)
Y.V. Melelera, N.A. Redy Chev, G.F. Nevikor, Inorg. Mater. 43(5), 455 (2007)
D.P. Padiyam, A. Marikani, K.R. Murali, Mater. Chem. Phys. 78, 51 (2002)
R.W. Glew, Thin Solid Films 46, 59 (1977)
N.J. Suthan Kissinger, M. Jayachandran, K. Perumal, C. Sanjeeviraja, Bull. Mater. Sci. 30, 547 (2007)
S.M. Rashwan, S.M. Abdul-Wahab, M.M. Mohammed, J. Mater. Sci. Mater. Electron. 18, 575 (2007)
S.S. Kale, H.M. Pathan, C.D. Lokhande, J. Mater. Sci. 40, 2635 (2005)
H.M. Pathan, C.D. Lokhande, Bull. Mater. Sci. 27, 85 (2004)
M. Ichimura, N. Sato, A. Nakamura, K. Takeuchi, E. Arai, Phys. Status Solid A 193, 132 (2002)
P.P. Hankare, P.A. Chate, D.J. Sathe, B.V. Jadhav, J. Alloys Compd. 503, 220 (2010)
N. Gopakumar, P.S. Anjana, P.K. Vidyadharan Pillai, J. Mater. Sci. 45, 6653 (2010)
C.S. Shahane, D.S. Sutrve, L.P. Deshmukh, Ind. J. Pure Appl. Phys. 34, 153 (1996)
N. Kumar, V. Sharma, U. Parihar, R. Sachdeva, N. Padha, C.J. Panchal, J. Nano Electron. Phys. 3, 117 (2011)
J. Barden, F.J. Blutt, L.H. Hall, in Proceedings of the Photoconductivity Conference, eds. by R. Brechepride Russel, E. Hahn (Wiley, New York, 1975)
A.V. Shaikh, R.S. Mane, H.M. Pathan, B.K. Min, O.S. Joo, S.H. Han, J. Electroanal. Chem. 615, 175 (2008)
P.P. Hankare, S.D. Delekar, P.A. Chate, M.R. Asabe, V.M. Bhuse, A.S. Khomane, K.M. Garardkar, B.D. Sarwade, J. Phys. Chem. Solids 67, 2506 (2006)
P.T. Landsberg, Recombinations in Semiconductors (Cambridge University, Cambridge, 1991)
Y.G. Gudange, N.G. Deshpande, A.A. Sagade, R.P. Sharma, S.M. Pawar, C.H. Bhosale, Bull. Mater. Sci. 30(4), 321 (2007)
F.S. Alamuti, J.R. Jennings, M.A. Hossain, L.Y.L. Yung, Q. Wang, Phys. Chem. Chem. Phys. 15, 4767 (2013)
G. Zhang, S. Finefrock, D. Liang, G.G. Yadav, H. Yang, H. Fang, Y. Wu, Nanoscale 3, 2430 (2011)
A.A. Yadav, E.U. Masumdar, Electrochim. Acta 56, 6406 (2011)
Acknowledgments
The authors V.M.B. and M.L.G. are grateful to the Director of Higher Education, Pune, Maharashtra for giving permission to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaur, M.L., Hankare, P.P., Garadkar, K.M. et al. CdSe thin films: morphological, optoelectronic and photoelectrochemical studies. J Mater Sci: Mater Electron 25, 190–195 (2014). https://doi.org/10.1007/s10854-013-1572-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-013-1572-9