Abstract
Cadmium selenide (CdSe) thin films of high crystalline quality on glass substrate have been prepared by chemical bath deposition technique from an aqueous bath containing tetramine cadmium and sodium selenosulphate. Structural analysis using XRD shows that the film is single phase, crystallized in hexagonal structure with preferred growth in (111) direction. The energy band gap calculated from the absorption spectra of unannealed CdSe thin films shows an optical band gap of 1.8 eV and absorption coefficient near band edge (α)—0.58 × 105 cm−1. The conductivity of CdSe thin films is n-type.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CdSe has been emerged as an important II–VI compound because of its exceptional opto electronic properties in the visible range. It offers great potential for the fabrication of solar cells, photo detectors, light emitting diodes, nanosensors, biomedical imaging devices, and other optoelectronic devices [1, 2]. It has a direct band gap (1.74 eV) with high absorption coefficient near the band edge, which allows its use in thin film devices and it is especially interesting for application in solar hybrid systems [3].
The electrical and optical properties of CdSe thin films for finding new applications are very sensitive to deposition conditions and to the technique used. Therefore, the study of the properties of CdSe with respect to different growing as well as ambient conditions is a matter of high importance. CdSe thin films has been prepared using a variety of methods including sputtering, spray pyrolysis, electrodeposition, etc. [4–6]. All these methods require sophisticated instruments and are costly. Of all the thin film deposition methods, chemical bath deposition (CBD) is the simplest one that offers great scope for large area fabrication. The CBD technique has been used for many years to prepare thin films of chalcogenide semiconductors and other kind of materials. This technique has been extensively used for the preparation of CdS thin films because of its application as a window layer material in solar cell fabrication [7]. CBD technique was used by several researchers for the deposition of CdSe thin films [8–10]. But the film prepared was not single phase and requires post-deposition treatments. In this paper we report the preparation and characterization of single phase, solar grade CdSe thin films by CBD technique by optimizing the process parameters.
Experimental procedure
CdSe thin films have been prepared by CBD technique. The principle underlying the deposition of CdSe thin films have been discussed by Chopra and Das [4]. It is based on the slow release of Cd2+ and Se2− ions in aqueous basic path (pH > 10) and the subsequent condensation of these ions on the substrate suitably mounted in the bath. The availability of Cd2+ ions is governed by the following dissociation of equilibrium.
The reduction of Na2SeSO3 to elemental Se was through the following reaction in the aqueous ammoniacal medium.
The Cd2+ ions released in the bath and the selenium ions condense to give CdSe solid phase provided that the ionic product is greater than the solubility product (K sp) of the compound. The solubility product of CdSe is very low and hence even very low concentration of Cd2+ and Se2− ions are sufficient to yield the solid phase. The rate of growth of the film depends on the temperature, pH value, and the relative concentration of Cd2+ and Se2− ions in the reaction mixture. By controlling these parameters uniform films of desired thickness can be grown.
The films were deposited on commercial quality glass slides (75 × 25 × 1.2 mm3). These slides were first dipped in hot trichloroethylene for 10 min and then in acetone to remove oily impurities adsorbed on the substrate surface. Slides were then cleaned in detergent, chromic acid, and distilled water. Finally it was dipped in acetone and dried.
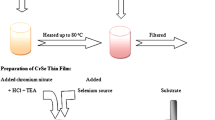
Chemical bath were constituted from 0.2 M solutions of CdCl2 and sodium selenosulphate. Sodium selenosulphate was prepared as follows: Elemental selenium in the form of fine powder was added to an aqueous solution of sodium sulphite heated to 90 °C. The solution was stirred well until all the selenium was dissolved. It was then cooled to room temperature and filtered to get a clear solution of Na2SeSO3. The chemical bath was prepared by the following sequence of addition of reactants: 20 mL of solution, few drops of triethanolamine, 30% NH4, and 20 mL of Na2SeSO3. The pH of the solution was adjusted to be >10, by adding excess ammonia. The solution was thoroughly stirred using a magnetic stirrer and the cleaned glass slides were supported vertically against the walls of the beaker. Deposition was completed in 2 h time at a temperature of 70 °C. After deposition the films were removed from the bath, washed in distilled water and dried.
The thickness of the film was estimated by gravimetric method using microbalance and by thickness prolifometer (Talysurf, CLI 1000). The CLA gauge in the range 311 μm–22 pm is used for the thickness measurement. The crystal structure of the samples was studied by recording the XRD spectra using the X-ray Diffractometer (Rigaku model D max/2C, Japan) using Cu Kα radiation (λ = 0.15046 nm) in the 2θ range 20°–60° at the scan rate of 4°/min. For taking XRD spectra, samples of thickness 0.75 μm were obtained by repeated dipping. The microstructure and surface morphology was analyzed by taking Scanning Electron Micrograph using SEM (model S-2400 Hitachi) for different magnifications.
Optical characterization was done by recording absorption spectra of the sample using a double beam spectrophotometer (Hitachi-220). Spectra were recorded in the wavelength range 500–900 nm. A blank glass slide was placed in one of the sample compartments to compensate for the absorption loss in the glass substrate. From the absorbance spectra the absorption coefficient for different wavelengths were calculated and a graph was plotted with α vs hν. The conductivity type was determined by hot probe method.
Results and discussions
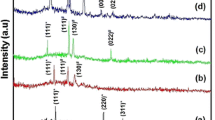
The thickness of the film in a single dipping estimated by gravimetric method and thickness prolifometry is 0.34 and 0.4 μm, respectively. Figure 1a–c shows the XRD spectra of samples unannealed (prepared at 70 °C), annealed at 150 °C, and annealed at 250 °C, respectively. All the XRD spectra shows the characteristic peaks of CdSe at angles 25° and 42° which corresponds to diffraction from (111) and (220) planes of ccp (zinc blend) structure, respectively, when compared with the standard JCPDS file No. (ICDD 19-0191). The peaks are broad probably due to the fineness of grains. The sample at 150 °C shows an additional characteristic peak which correspond to diffraction from (311) plane of cubic CdSe phase. The crystallinity of the film increased with annealing temperature. An additional peak around 32 °C indicates the occurence of a new phase Cd1−x Se x which is present for the sample annealed at 150 °C.
The microstructure of the film plays a vital role on the properties of solar cells fabricated. The SEM micrograph of CdSe thin films (a) unannealed and (b) annealed at 150 °C is shown in Fig. 2a, b. The unannealed sample is nonuniform with two layers and agglomeration of microcrystallites is seen. The grain size is small which is usually the case with unannealed sample. The average grain size estimated is about 0.45 μm. The uniformity of film increased with annealing and grain size improved to about 1 μm.
α vs hν graph of CdSe thin films unannealed and annealed at different temperatures are shown in Figs 3, 4, 5, and 6, respectively. The sharp rise in the absorption suggests that it is direct band gap semiconductor. Table 1 shows the variation of band gap energy with annealing temperature of CdSe films. The unannealed sample has the highest band gap energy of 1.83 eV. The sample annealed at 250 °C shows the lowest band gap of 1.38 eV. The decrease in band gap energy with annealing temperature shows that the film becomes more uniform with annealing. The sample annealed at 150 °C shows two slopes—a direct band gap of 1.75 eV which corresponds to that of CdSe and an indirect band gap of 1.3 eV that corresponds to the additional phase Cd1−x Se x . The presence of additional phase Cd1−x Se x is again confirmed. The direct band gap energy of CdSe sample is comparable with that reported by Mangalhara et al. [11] (1.74 eV). The absorption coefficient near the band edge is 0.58 × 105 cm−1.
The CdSe thin film has n-type conductivity. The resistivity of the sample is very high (>200 MΩ). This is quite obvious since the film is highly stoichiometric.
Conclusions
Cadmium selenide thin films have been prepared by CBD technique. The process parameters like temperature, pH values, and annealing temperature are optimized to get uniform films on glass substrate. XRD spectra indicate the single phase nature of CdSe films. CdSe film annealed at 150 °C shows an additional phase Cd1−x Se x . The unannealed CdSe films have band gap energy of 1.8 eV and with increase in annealing temperature the band gap energy decreases. The results show that CdSe thin films prepared by CBD technique have high structural and optical characteristics, which are viable candidates for application in solar energy conversion devices. Experiments are going on in our laboratory to convert these films into p-type by doping so that p–n hetrojunctions can be grown completely by CBD technique.
References
Hendry E, Koeberg M, Wang F, Zhang H, de Mello Donega C, Vanmaekelbergh D, Bonn M (2006) Phys Rev Lett 96:057408
Schaller RD, Petruska MA, Klimov VI (2005) Appl Phys Lett 87:253102
Vorobiev Yu, Gonzalez-Hernandez J, Vorobiev P, Bulat L (2006) Sol Energy 80:170
Chopra KL, Das SR (1983) Thin film solar cells. Plenum Press, New York, London
Cachet H, Cortes R, Froment M, Etcheberry A (2000) Thin Solid Films 361–362:84
Ju ZG, Lu YM, Zhang J, Wu XJ, Liu KW, Zhao DX, Zhang ZZ, Li BH, Yao B, Shen DZ (2007) J Cryst Growth 307:26
Nemec P, Nemec I, Nahalkova P, Nemcova Y, Knizec K, Maly P (2002) J Cryst Growth 240:484
Mondal AK, Chauduri TK, Pramanik P (1983) Sol Energy Mater 7:431
Pathan HM, Sankapal BR, Desai JD, Lokhande CD (2003) Mater Chem Phys 78:11
Kale RB, Lokhande CD (2005) Semicond Sci Technol 20:1
Mangalhara JP, Thangaraj R, Agnihotri OP (1988) Bull Mater Sci 10:333
Acknowledgements
The authors are grateful to University Grants Commission, New Delhi for the financial assistance for this work. The authors would like to thank Mr. C. V. Muraleedharan and Ms. Leena Joseph of Bio Medical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences, Trivandrum for thickness measurement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gopakumar, N., Anjana, P.S. & Vidyadharan Pillai, P.K. Chemical bath deposition and characterization of CdSe thin films for optoelectronic applications. J Mater Sci 45, 6653–6656 (2010). https://doi.org/10.1007/s10853-010-4756-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4756-1