Abstract
A TiO2 film was prepared on Pt/Ti/SiO2/Si substrate by a laser chemical vapor deposition method. The rutile TiO2 film with pyramidal grains and columnar cross-section was obtained at a high deposition rate (R dep = 11.4 μm h−1). At 300 K and 1 MHz, the dielectric constant (ε r) and loss (tanδ) of the TiO2 film were about 73.0 and 0.0069, respectively. The electrical properties of TiO2 film were investigated by ac impedance spectroscopy over ranges of temperature (300–873 K) and frequency (102–107 Hz). The Cole–Cole plots between real and imaginary parts of the impedance (Z′ and Z′′) in the above frequency and temperature range suggested the presence of two relaxation regimes that were attributed to grain and grain boundary responses. The ionic conduction in the rutile TiO2 film was dominated by the oxygen vacancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The rutile TiO2 films are widely investigated because they have many applications such as capacitor, sensors, antireflection coatings and corrosion-resistant barriers [1–5]. The dielectric constant (ε r) of rutile TiO2 crystal is anisotropic and has values of 170 in the c direction and 89 perpendicular to the c direction, which indicates that TiO2 film has the possibility application to future ultra-large-scale dynamic random access memory (DRAM) [6, 7]. TiO2 films have been prepared by many methods, such as sputtering, conventional chemical vapor deposition, sol–gel method, type-casting and laser chemical vapor deposition (LCVD) [8–15]. Among these methods, the high-quality film were prepared by LCVD with high deposition rate (R dep). Goto et al. [15] prepared TiO2 films on alumina plates with a mixture phase of rutile and anatase by LCVD with very high R dep (about 3,000 μm h−1). Recently, Gao et al. [16] reported that the rutile TiO2 films were deposited on Yttria-stabilized zirconia (YSZ) plates by LCVD, and the R dep was about 240 μm h−1. However, they didn’t study the dielectric property of the TiO2 films. Numerous research indicated that the dielectric and electrical properties of TiO2 films were dependent on their microstructure [17, 18]. The films prepared by LCVD always had the characteristic columnar cross-section [19–23]. It is necessary to investigate the electrical properties of the rutile TiO2 film prepared by LCVD.
To investigate the dielectric property and electrical conductivity of the rutile TiO2 film, a Pt/Ti/SiO2/Si substrate, which had good electrical conductivity and thermal stability, was used to replace the ceramic substrates in references [15] and [16]. In this study, TiO2 film was prepared on Pt/Ti/SiO2/Si substrate by LCVD, and the dielectric properties and electrical conductivity of TiO2 film were investigated.
2 Experimental
The TiO2 film was prepared on Pt/Ti/SiO2/Si substrates by LCVD with a continuous-wave Nd:YAG laser (wavelength: 1,064 nm). A schematic of the LCVD apparatus has been reported elsewhere [19, 20]. The substrate was put on a hot stage with a pre-heating temperature (T pre) of 773 K. A thermocouple was inserted at the bottom side of the substrate to measure the deposition temperature (T dep). In this study, T dep was 858 K. A laser beam, 14 mm in diameter, was introduced through a quartz window to irradiate the whole substrate. The laser power (P L) was 48 W. The titanium diisopropoxy-dipivaloylmethanate (Ti(Oi–Pr)2 (DPM)2, Toshima Manufactory) precursor was heated at 423 K, and the vapor was carried into the chamber with Ar gas. The O2 gas was separately introduced into the chamber through a double-tube gas nozzle. The total pressure (P tot) in the CVD chamber was held at 600 Pa. The deposition was conducted for 1,800 s. Details of the deposition conditions are listed in Table 1.
The crystal structure was analyzed by X-ray diffraction (XRD, Rigaku RAD—2C) using CuKα X-ray radiation. The surface and cross section microstructures were observed by a scanning electron microscope (SEM, Hitachi S-3100H). To measure the impedance spectroscopy, the top electrode was fabricated on the surface of the TiO2 film from Au paste, which was dried and decomposed by gradually heating to 1,073 K. Impedance spectroscopic measurements were performed using an impedance/grain-phase analyzer (Hewlett Packard 4194) at an ac driving voltage of 10 mV in the temperature range from 300 to 873 K over the frequency range of 102–107 Hz.
3 Results and discussions
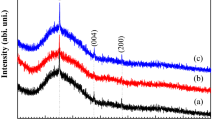
Figure 1 shows the XRD pattern of TiO2 film. It was indexed to the rutile TiO2 phase (JCPDS 21-1276). The single-phase TiO2 film was obtained. Figure 2 displays the cross-sectional and surface SEM images. As shown in Fig. 2a, the TiO2 film had columnar cross-section, and the film thickness was about 5.7 μm that indicated the R dep was 11.4 μm h−1, which was slower than these reported by Goto et al. [15] and Gao et al. [16] due to the low T Ti in this study. However, the present R dep was still much higher than that prepared by other methods [8–13]. The surface consisted of pyramidal grains. Figure 2c shows the schematic of crystal structure, which was illustrated by VESAT, a three-dimensional visualization system [24]. In the rutile crystal structure, the basic building block consists of a titanium atom surrounded by six oxygen atoms in a more or less distorted TiO6 octahedral configuration. The two bonds between the titanium and the oxygen atoms at the apices of the octahedron are slightly longer [1]. The pyramidal grains in the surface of TiO2 film, as shown in Fig. 2b, were attributed to the stacked TiO6 octahedral.
Figure 3 presents the temperature dependence of dielectric properties of the TiO2 film in the temperature range from 300 to 873 K at 1 MHz. At 300 K, the ε r and dielectric loss (tanδ) of the TiO2 film were about 73.0 and 0.0069, respectively. The ε r value in this work was close to those of the rutile TiO2 crystal perpendicular to the c direction and the rutile TiO2 film prepared by Kim et al. [6, 7] which also indicated the single-phase rutile TiO2 film was prepared by LCVD. With increasing temperature, the ε r slightly decreased, and then increased. A peak around 753 K was observed, which indicated that there was a phase transition. Generally, the transition temperature is about 1,173 K. However, it also depends on pressure, stress, contaminants, oxygen deficiency, etc. [25]. The tanδ increased slightly with increasing temperature from 300 to 753 K. As temperature was beyond 753 K, the tanδ abruptly increased due to the phase transition.
Figure 4 depicts the frequency dependence of the imaginary part of impedance (Z′′) at different temperatures. At 300 K, there was no peak in the measured frequency range, since the film had high resistance. With increasing the temperature, the resistance decreased and the peaks (Z′′ max) with characteristic frequency maximum (f max) were observed due to the grain effect. The peaks were suggestive of relaxation processes with a spread of relaxation time, which were temperature dependent. As temperature increased, the f max shifted to higher frequency and the Z′′ max decreased due to the decrease of resistance of TiO2 film.
Figure 5a depicts the Cole–Cole plots of complex impedance data (the imaginary part Z′′ and the real part Z′) at different temperatures. The arrows in Fig. 5a showed the direction of increasing frequency. At low temperatures, an almost straight line with big slope was observed due to high resistance. With increasing temperature, the resistance decreased and the impedance semicircles were observed. Figure 5b shows the enlarged Cole–Cole plot obtained at 870 K. There were two semicircles, which suggested the presence of two relaxations that were attributed to grain and grain-boundary responses. To analyze and interpret the Cole–Cole plots, an equivalent circuit model comprising two parallel RC elements in series was established as shown in Fig. 5c. The low-frequency semicircle corresponded to the grain-boundary response (R gb C gb) and the high-frequency one corresponded to the grain response (R g C g). Figure 5b showed the good agreement between the experimental data (triangular patterns) and the theoretical curve (dotted line, generated from ZVIEW software).
Figure 6 shows the ac electrical conductivity plots as a function of frequency at different temperatures. At 562 K, the conductivity–frequency curve had a frequency-independent dc component at low frequency (100–104 Hz) and a frequency-dependent ac component at high frequency (104–107 Hz). With increasing temperature, the curves tended to flatten. Salam et al. [26] analyzed the frequency dependent ac electrical conductivity data and proposed the frequency dependence of electrical conductivity to be of the form
where σ(ω) is the total conductivity, σ(0) is the frequency-independent conductivity, and the coefficient A and exponent n are temperature and material dependent. The frequency-independent plateau at the low frequency was associated with the term σ(0) contributing to dc conductivity. As frequency increased, two dispersion regions appeared for all temperatures. The mid-frequency dispersion (A 1 ω n1) was associated with the grain boundaries, while the high-frequency one (A 2 ω n2) with the grains [27, 28]. The electrical conductivity of grain (σ g) was obtained from the grain resistance (R g) using the relation.
where t is the film thickness and A is the electrode area. The temperature dependence of σ g in Arrhenius format was described by
where σ 0 is the pre-exponential factor, E a the activation energy for the conduction process, k the Boltzmann constant and T the absolute temperature. The Arrhenius plot of σ g is shown in Fig. 7. The values of E a for the grain conduction at high-temperature region (744–873 K) and low-temperature region (555–744 K) were 1.06 and 0.69 eV, respectively. This result indicated that the different conductive mechanisms dominated in the different temperature regions. TiO2 can be hardly oxidized but easily reduced to TiO2−x contains extra Ti and/or missing O atoms, which suggests that the dominant native point defects should be the oxygen vacancy V O and the titanium interstitial Ti I [29]. Iddir et al. [30] calculated the diffusion barriers of V 2+O defect. They investigated the diffusion of V 2+O by moving the oxygen vacancy along three paths (path A along [001], B along \( [1\overline{1} 0] \) and C from the plane to the apex position of the octahedron) in the TiO6 octahedron. The diffusion barriers along path A, B and C were 1.77, 0.69 and 1.10 eV, respectively. According to Fig. 2b, the columnar cross-section consisted of the stacked TiO6 octahedral. The E a of 0.69 eV indicated that the migration of oxygen vacancy along path B dominated at the temperature range of 555–744 K. As the temperature was near 744 K, the phase transition happened. And at the temperature range of 744–873 K, the E a of 1.06 eV was close to the 1.10 eV, which indicated that the migration of oxygen vacancy along path C dominated. In the present study, the ionic conduction in the rutile TiO2 film was dominated by the oxygen vacancies.
4 Conclusions
The rutile TiO2 film was prepared on Pt/Ti/SiO2/Si substrate with high R dep (11.4 μm h−1) by LCVD. The TiO2 film consisted of pyramidal grains with columnar cross-section. At 300 K and 1 MHz, the ε r and tanδ of the TiO2 film were about 73.0 and 0.0069, respectively. The E a of the grain conduction in the TiO2 film at high-temperature region (744–873 K) and low-temperature region (555–744 K) were 1.06 and 0.69 eV, respectively. The ionic conduction in the rutile TiO2 film was dominated by the oxygen vacancies. At 555–744 K, the migration of oxygen vacancy along path B dominated at the temperature range. And the migration of oxygen vacancy along path C dominated at 744–873 K.
References
U. Diebold, Surf. Sci. Rep. 48, 53–229 (2003)
M. Kadoshima, M. Hiratani, Y. Shimamoto, K. Torii, H. Miki, S. Kimura, T. Nabatame, Thin Solid Films 424, 224–228 (2003)
D.A.H. Hanaor, C.C. Sorrell, J. Mater. Sci. 46, 855–874 (2011)
A. Fujishima, X. Zhang, D.A. Tryk, Surf. Sci. Rep. 63, 515–582 (2008)
A. Fujishima, K. Honda, Nature 238, 37–38 (1972)
R.A. Parker, Phys. Rev. 124, 1719–1722 (1961)
S.K. Kim, W. Kim, K. Kim, C. Hwang, J. Jeong, Appl. Phys. Lett. 85, 4112–4114 (2004)
P. Alexandrov, J. Koprinarova, D. Todorov, Vacuum 47, 1333–1336 (1996)
N. Rausch, E.P. Burte, J. Electrochem. Soc. 140, 145–149 (1993)
Q. Zhang, G.L. Griffin, Thin Solid Films 263, 65–71 (1995)
J.Y. Kim, H.S. Jung, J.H. No, J.R. Kim, K.S. Hong, J. Electroceram. 16, 447–451 (2006)
I. Oja, A. Mere, M. Krunks, R. Nisumaa, C.H. Solterbeck, M. Es-Souni, Thin Solid Films 515, 674–677 (2006)
C. Sheng, D. Fatih, Int. J. Appl. Ceram. Technol. 8, 1363–1373 (2011)
M. Gao, A. Ito, R. Tu, T. Goto, Key Eng. Mater. 508, 287–290 (2012)
T. Goto, T. Kimura, Key Eng. Mater. 317–318, 495–500 (2006)
M. Gao, A. Ito, R. Tu, T. Goto, Key Eng. Mater. 508, 279–282 (2012)
C. Demetry, X. Shi, Solid State Ionics 118, 271–279 (1999)
C. Sheng, P. Vladimir, D. Fatih, J. Am. Ceram. Soc. 93, 3031–3034 (2010)
D. Guo, T. Goto, C. Wang, Q. Shen, L. Zhang, J. Mater. Sci.: Mater. Electron. 23, 897–900 (2012)
D. Guo, T. Goto, C. Wang, Q. Shen, L. Zhang, Mater. Lett. 70, 135–137 (2012)
D. Guo, A. Ito, T. Goto, R. Tu, C. Wang, Q. Shen, L. Zhang, J. Mater. Sci. 47, 1559–1561 (2012)
D. Guo, T. Goto, C. Wang, Q. Shen, L. Zhang, Appl. Surf. Sci. 258, 7860–7863 (2012)
D. Guo, T. Goto, C. Wang, Q. Shen, L. Zhang, J. Electron. Mater. 41, 689–694 (2012)
K. Momma, F. Izumi, J. Appl. Crystallogr. 41, 653–658 (2008)
J.M. Criado, C. Real, J. Soria, Solid State Ionics 32–33, 461–465 (1989)
F. Salam, J.C. Giuntini, S.S. Soulayman, J.V. Zanchetta, Appl. Phys. A 63, 447–452 (1996)
A. Pelaiz-Barranco, M.P. Gutierrez-Amador, A. Huanosta, R. Valenzuela, Appl. Phys. Lett. 73, 2039–2041 (1998)
N. Ortega, A. Kumar, P. Bhattacharya, S.B. Majumder, R.S. Katiyar, Phys. Rev. B 77, 014111 (2008)
A.M. Asaduzzaman, P. Kruger, J. Phys. Chem. C 114, 19649–19652 (2010)
H. Iddir, S. Ogut, P. Zapol, N.D. Browning, Phys. Rev. B 75, 073203 (2007)
Acknowledgments
This work was supported in part by Global COE Program of the Materials Integration, Tohoku University, and by the International Science and Technology Cooperation Program of China (Grant No. 2009DFB50470).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, D., Ito, A., Goto, T. et al. Preparation of TiO2 thick film by laser chemical vapor deposition method. J Mater Sci: Mater Electron 24, 1758–1763 (2013). https://doi.org/10.1007/s10854-012-1008-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-012-1008-y