Abstract
Y2O3:Eu3+ nano phosphors (about 65 nm in size) were prepared via co-precipitation method by using co-polymer NIPAM/AAc as a template with narrow size distribution. The aim of the paper is to indicate the co-polymer influence on size and luminescence characteristics of nano phosphors. In this respect, phase content and morphology were characterized by X-ray diffraction and transmission electron microscopy. All the powders were well crystallized and the particles were clearly spherical with good dispersibility. Luminescence measurements were used to investigate the role of precursor in powders and the utmost luminescence intensity was observed at 4 mol% of Eu3+ while the quenching concentration of Eu3+ ions has occurred at 5 mol%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Yttrium oxide is a suitable host for many lanthanide oxides because of the resemblance between their ionic radius and crystal structure and doping most of these oxides produce variety of phosphors with different fluorescent spectra i.e. green for Tb, red for Eu, Yellow for Dy, blue for Tm [1, 2]. Among these phosphors, Y2O3:Eu3+ is well-known red phosphor typically used in field emission display (FED) devices, cathode ray tubes (CRT) applications, trichromatic fluorescent lamps and plasma display panels [3–7]. The fluorescence property of Y2O3:Eu3+ strongly depends on its structure. Commercial type of this phosphor has the cubic structure because the cubic structure with space group: Ia3 (206) possesses great potential of fluorescence intensity compare to the monoclinic structure with space group of C2/m(12) [8–10]. The cubic lattice contains two crystallographic sites in which the M3+ ions are found as outlined in Fig. 1, one has C2 symmetry and the other S6 symmetry; both sites are six coordinate and are present the ratio of 3:1 [11–13]. In particular, europium (III) has five narrow emission bands corresponding to the 5D0 → 7Fi transitions where i = 0, 1, 2, 3 and 4. The strongest transition, 5D0 → 7F2 occurs at approximately 610 nm, which is characteristic of red fluorescence of Eu3+ [14].
Some qualities including high purity level of host structure, doping concentration of Eu and particle size have the most important influences on industrial fabrication of nanophosphor Y2O3:Eu3+. In order to produce a nanophosphor with high quality, a variety of synthesis methods has been studied such as: sol–gel [3], spray pyrolysis [9], hydrothermal decomposition [15], chemical precipitation of precursors in aqueous or organic solutions and addition of modifying agents such as: EDTA and polymers as a template [16–19]. In the present work, mono-dispersed nanophosphor Y2O3:Eu3+ particles are synthesized in urea aqueous solution by homogeneous precipitation method. The effect of experimental variables, such as: presence of NIPAM/AAc co-polymer as a template, size distribution and europium concentrations were characterized in terms of luminescence properties.
2 Experimental procedures
2.1 Starting materials
The entire chemical materials which are used in this work were obtained from Merck Fine Chemicals.
2.2 Synthesis of europium doped yttria
Y2O3:Eu3+ was prepared via homogeneous precipitation method by utilizing NIPAM/AAc as a template and urea as a precipitant while the preparation of the copolymer is described perfectly in our previous study [20]. In order to prepare Y2O3 phase with the copolymer a known amount of Y(NO3)3
and Eu(NO3)3 (2–8 mol% with respect to Y) were prepared by dissolving Eu2O3 and Y2O3 in dilute HNO3. Then, an excess amount of urea is dissolved in the vessel and mixed with the above solutions thoroughly. Then 30 ml of NIPAM/AAc copolymer was added to the solution at adjusted pH of 3 to complete the precipitation reaction and the temperature is raised up to 85 °C for 1 h. The resulting precipitates were separated and washed with ethanol and to dry the precipitates, it was put into oven and pre-heated at 100 °C overnight. Finally, calcinations treatment was carried out in a muffle furnace for 3 h at 980 °C. Also to compare the physico-chemical effects of the copolymer, a sample without copolymer was prepared and the precursor solutions containing Y(NO3)3 and Eu2O3 (2 wt%) in dilute HNO3were used.
2.3 Physical measurements
The crystal structure of the Y2O3 phase was identified by X-ray diffraction (XRD, Siemens D-500; Cukα radiation at 40 kV and 30 mA). The particle size and distribution of prepared samples were analyzed using a Malvern Zeta Sizer after the preparation. The samples had to be diluted in water and they were also inserted in an ultrasonic bath for 10 min. Microstructure of the samples was observed by scanning electron microscope (SEM) Cambridge at a voltage of 25 kV sputtered by gold. The emission and excitation spectra were obtained using a Perkin Elmer LS-5 Luminescence spectroscopy.
3 Result and discussion
3.1 X-ray powder diffraction analysis
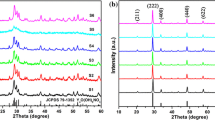
X-ray diffraction (XRD) patterns of Y2O3/Eu3+ prepared at 980 °C is shown in Fig. 2. As it is clear, there is no evidence of appearing two assigned peaks of monoclinic phase at 2θ = 32.1° and 29.78° and all the peaks are in good agreement with powder data in JCPDS card No. 25-1011. According to that, the samples are cubic crystalline Y2O3:Eu3+ without impurity [21]. In addition, comparing XRD patterns of samples (a and b) shows that cubic phase of Y2O3 is formed completely and both samples have the similar crystallinity regardless of NIPAM/AAc copolymer. Also, it could indicate that the doped Eu3+ ions have entered the Y3+ sites completely because usually, doping into space among crystal lattice will cause lattice distortion and then origin the change of the cell parameters.
3.2 Luminescent analysis
3.2.1 Particle size effect on emission spectra
The proposed mechanism for the formation of the phosphor precursor of Y2O3:Eu3+ explains the cause of decreasing the particle size in presence of NIPAM/AAc co-polymer [22, 23]. The carboxyl groups incorporated into the molecule of the copolymer by adding the AAc; give it a negative surface charge. On the other hand, decomposition of urea increases pH and the hydrolyzed yttrium ions by water will combine with anionic groups on the surface of the copolymer micro-gel, meantime hydroxyl carbonate precursor forms and links to the copolymer. The final particle is a copolymer coated by the hydroxyl carbonate precursor of the phosphor. After annealing treatment the copolymer decomposed and the final Y2O3:Eu3+ nanophosphor formed. Thus the particle of the sintered phosphor is more regular and the size is small due to the presence of the copolymer. The TEM micrographs (Fig. 3a, b) show the particles morphology of the obtained Y2O3:Eu3+ nanoparticles with/without copolymer precursor, respectively. It can be seen that both samples have a non-agglomerated spherical shape with clear particle boundaries and were nano-sized. Fig. 4a, b illustrate mean particle size of 65 and 170 nm for both samples. It is clear that the sample with co-polymer contains particles portion with diameters from 55 to 65 nm while the sample without the copolymer (Fig. 4b) owns the particles portion with diameters from about 165 to 173 nm. It is obvious that NIPAM/ACC co-polymer has influence on the growth surroundings of the particle and the variation of particle sizes is a direct function of that [24]. This co-polymer can prevent the agglomeration of nanoparticles by reducing the surface tension and also prevents particles from contacting with each other by steric-hindrance effect. So, the particles diameter can be restricted to less than 65 nm.
As shown in Fig. 5 the emissions spectrum patterns under 254 nm excitation for the prepared samples with/without copolymer were analogous to each other and there is no position change of the emission peaks for both powders, but the intensity is appeared to be dissimilar according to two different type of synthesis media. The spectra are composed of notable peaks at 582, 587, 592, 598 and 610 nm. The transitions ranging from 582 to 598 nm are assigned to the site selection of europium in host matrix and occupancy of the symmetric S6 site by the Eu3+ ion [25–30], while the strongest one at 610 nm is attributed to 5D0 → 7F2 transition in the C2 site of Y2O3 [27, 31]. It was reported that among two Y3+ sites in cubic Y2O3; 75 % of these have C2 symmetry and 25 % are sites with S6 inversion symmetry [13, 32]. The emission spectra clearly shows that precipitation process with the NIPAM/AAc co-polymer alike traditional synthesis method, leads the europium cations to occupy C2 sites too. Furthermore, synthesised sample has higher intensity than sample without co-polymer, because Eu3+ ions enter the Y2O3 crystal lattice more easily in the presence of NIPAM/AAc co-polymer due to decrease of particle size. Thus, the activator concentration is higher although the same amount of Eu3+ is doped in the Y2O3 host in both methods.
3.2.2 Europium concentration effect on emission spectra
In order to determine the effect of the Eu3+ concentration on luminescence intensity, different concentrations (Eu3+/Y3+ mol ratio: 0.02, 0.03, 0.04, 0.05, 0.06, 0.07 and 0.08) of Eu3+ doped Y2O3 nanoparticles were synthesized via NIPAM/AAc co-polymer route. The emission intensity of Y2O3:Eu3+ with respect to the doping concentration of the Eu ions is shown in Fig. 6. Initially, the emission intensity increased with increasing Eu/Y up to Eu/Y = 0.04 mol ratio, and then started reducing with increasing Eu3+ concentration which is due to critical quenching concentration of Eu3+. The critical quenching concentration is defined as the concentration at which the emission intensity begins to decrease during the increase of Eu3+ concentration [33]. The quenching effect occurs since the interaction between activator ions increases [34]. As stated in Sect. 3.2.1, there is an excellent probability of entering Eu3+ in both sites of S6 and C2 which generates strong interaction among activator sites. It means, by increasing Eu3+ concentration from 0.02 to 0.08 mol and enhancing the number of activator centers, the emission from one Eu center will be quenched by transferring excitation energy to another Eu center. This transformation between the centers would proceed till the excitation energy quenches, so the luminescence intensity would decrease. Thus, the critical quenching concentration of Eu3+ in prepared Y2O3:Eu3+ with co-polymer nanophosphor is 0.05 mol.
4 Conclusions
In conclusion, Y2O3:Eu3+ nano phosphor is prepared via a precipitation method using a NIPAM/AAc co-polymer successfully. This process proves to be very useful, simple and whilst pure cubic Y2O3:Eu3+ nano-powder with high homogeneity can be obtained. The results reveal that NIPAM/AAc copolymer has remarkably diminished the particle size from 165 to 65 nm while all the particles shaped clearly spherical and non-agglomerated. In comparison with the phosphors prepared by a conventional method, the emission intensity of Y2O3:Eu3+nanopowder revealed that the nature of co-polymer influences the luminescence characteristics of nano phosphors and doping Eu3+ ions at a suitable content (4 mol%) can greatly improve the luminescence intensity. In contrast, emission intensity reduced in the case of increasing Eu3+concentration and the quenching effect was observed at the concentration 5 mol% of Eu3+. All the results indicate that Y2O3:Eu3+ nanopowder prepared by using co-polymer NIPAM/AAc template is a promising red phosphor with high luminescence intensity.
References
J. Hao, S.A. Studenikin, M. Cocivera, J. Lumin. 93, 313 (2001). doi:10.1016/S0022-2313(01)00207-1
F. Vetrone, J.C. Boyer, J.A. Capobianco, A. Speghini, M. Bettinelli, Nanotechonology 15, 75 (2004). doi:10.1088/0957-4484/15/1/015
P.K. Sharma, M.H. Jilavi, V.K. Varadan, H. Shmidt, J. Phys. Chem. Solid 63, 171 (2002). doi:10.1016/S0022-3697(01)00124-X
N. Joffin, J. Dexpert-Ghys, M. Verelst, G. Baret, A. Garcia, J. Lumin. 113, 249 (2005). doi:10.1016/j.jlumin.2004.10.021
K. Pramod, P.K. Sharma, R. Nass, H. Schmidt, Opt. Mater. 10, 169 (1998). doi:10.1016/S0925-3467(97)00140-7
Y. Qi, L. Sun, M. Lee, B.I. Samuels, G.J. Exarhos, J. Lumin. 109, 85 (2004). doi:10.1016/j.jlumin.2004.01.085
B. Guoa, H. Yima, W. Hwanga, M. Nowellb, Z. Luoc, Particuology 9, 24 (2011). doi:10.1016/j.partic.2010.07.020
X. Qin, Y.G. Ju, S. Bernhard, N. Yao, J. Mater. Res. 20, 2960 (2005). doi:10.1557/JMR.2005.0364
D. Dosev, B. Guo, I.M. Kennedy, J. Aerosol Sci. 37, 402 (2006)
A. Camenzind, R. Strobel, F. Krumeich, S.E. Pratsinis, Adv. Powder Technol. 18, 5 (2007). doi:10.1163/156855207779768142
M.G. Paton, E.N. Maslem, Acta. crystallogr. 19, 307 (1965). doi:10.1107/S0365110X65003365
H. Ishibashi, K. Shimomoto, K. Nakahigashi, J. Phys. Chem. Solids 55, 809 (1994). doi:10.1021/jp0102522
M. Mitric, B. Antic, M. Balanda, D. Rodic, M.L. Napijalo, J. Phys. Condens. Matter 9, 4103 (1997)
B.C. Joshi, J. Non-Cryst. Solids 180, 217 (1995). doi:10.1016/0022-3093(94)00478-1
X.B. Hong, L. Yu, L. Yang, Z. Liu, G. Pan, S. Lu, X. Ren, Y. Lei, L. Fan, J. Phys. Chem. B 109, 15236 (2005). doi:10.1016/S1466-6049(00)00065-9
P.K. Sharma, M.H. Jilavi, H. Schmidt, V.K. Varadan, Int. J. Inorg. Matter 2, 407 (2000). doi:10.1016/S1466-6049(00)00065-9
X. Jing, T. Ireland, C. Gibbons, D.J. Barber, J. Silver, A. Vecht, G. Fern, P. Trowga, D.C. Morton, J. Electrochem. Soc. 146, 4654 (1999)
M.I. Martinez-Rubio, T.J. Ireland, J. Silver, G. Fern, C. Gibbons, A. Vecht, Electrochem. Solid-State Lett. 3, 446 (2000). doi:10.1002/chin.200049231
Z. Yongqing, Y. Zihua, D. Shiwen, Q. Mande, Z Jian Mater. Lett. 57, 2901 (2003). doi:10.1016/S0167-577X(02)01394-0
Sh Fadaie, M.M. Kashani-Motlagh, A. Maghsoudipour, B. Faridnia, Pigment Resin Technol. 39, 214 (2010). doi:10.1108/03699421011055527
S. Katagiri, N. Ishizawa, F. Marumo, Powder Diffr. 8, 60 (1993)
J. Silver, M.I. Martinez-Rubio, S. Gebretensae, G.R. Fern, M.J. Snowden, R. Withnall, SID Symp. Dig. Tech. Pap. 33, 393 (2002)
S. Zhong, J. Chen, S. Wang, Q. Liu, Y. Wang, S. Wang, J. Alloy. Compd. 493, 322 (2010). doi:10.1016/j.jallcom.2009.12.092
X.H. Liu, J. Yang, L. Wang, X.J. Yang, L.D. Lu, X. Wang, Mater. Sci. Eng. A 289, 241 (2000). doi:10.1016/S0921-5093(00)00901-1
F. Parasapour, D.F. Kelley, R.S. Williams, J. Phys. Chem. B 102, 7971 (1998). doi:10.1021/jp982628q
D.B.M. Klaassen, R.A.M. Van Ham, T.G.M. Van Rijn, J. Lumin. 40, 651 (1989). doi:10.1016/0022-2313(89)90096-3
M. Buijs, A. Meijerink, G. Blasse, J. Lumin. 37, 9 (1987). doi:10.1016/0022-2313(87)90177-3
A. Garcia-Murillo, C.L. Luyer, C. Garapon, C. Dujardin, E. Bernstein, C. Pedrini, J. Mugnier, Opt. Mater. 19, 161 (2002). doi:10.1016/S0925-3467(01)00214-2
R.E. Ouenzerfi, C. Goutaudier, M. Cohen-Adad, Th Panczer, G. Boulon, J. Lumin. 102, 426 (2003). doi:10.1016/S0022-2313(02)00580-X
V. Jubera, J.P. Chaminade, A. Garcia, F. Guillen, C. Fouassier, J. Lumin. 101, 1 (2003). doi:10.1016/S0022-2313(02)00335-6
R.B. Hunt, R.G. Pappalardo, J. Lumin. 34, 3133 (1985). doi:10.1016/0022-2313(85)90085-7
J. Silver, M.I. Martinez-Rubio, T.G. Ireland, R. Withnall, J. Phys. Chem. B 105, 7200 (2001). doi:10.1021/jp0102522
D. Wang, Q. Yin, Y. Li, M. Wang, J. Lumin. 97, 1 (2002). doi:10.1016/S0022-2313(01)00413-6
Shigeo SM (1990) Phosphor Handbook, Vol 99, CRC Press, New York, pp 108–109
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fadaie, S., Kashani-Motlagh, M.M., Maghsoudipour, A. et al. Effect of dopant concentration and particle size on fluorescence properties of Y2O3:Eu3+ derived in presence of NIPAM/AAc copolymer. J Mater Sci: Mater Electron 24, 974–978 (2013). https://doi.org/10.1007/s10854-012-0860-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-012-0860-0