Abstract
The Ln2/3Gd1/3TiNbO6 ceramic compositions are prepared through the solid state ceramic route. The compositions are calcined at 1250 °C and sintered in the range 1350–1435 °C. Structural analysis of the materials is done using X-ray diffraction analysis and vibrational spectroscopy. Surface morphology is examined by Scanning Electron Microscopy. Microwave dielectric properties such as dielectric constant (εr), quality factor (Q) and temperature coefficient of resonant frequency (Tf) are measured using cavity resonator method. The compositions have εr in between 46 and 41.8 and Tf in between +52 and +25 ppm/°C. By the substitution of Gd, the Tf is reduced considerably with a slight decrease in dielectric constant. Cerium based composition had additional reflections other than that of aeschynite structure. For Pr, Nd and Sm based systems, solid solutions were formed. UV visible spectrum of the representative composition is recorded and the band gap energy is estimated. Photoluminescence spectra of the samples are recorded and the transitions causing emissions are identified. The materials are suitable for microwave and optoelectronic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The advancement in microwave communication resulted in an increased demand for new materials suitable for microwave applications [1]. Dielectric Resonators (DR), the ceramic puck used for microwave communication have the advantage of more miniaturization, high quality factor than other techniques like transmission lines, micro strips and cavities, low cost and can easily be coupled to microwave integrated circuits [2]. Widespread research is going on in the field of DR as they have high dielectric constant (εr), high quality factor (Q) and small variation of resonant frequency with respect to temperature (Tf) [3]. It is interesting to note that most of the DR with good dielectric properties are complex oxides of titanium, niobium and tantalum. Komkov et al. [4] and Qi et al. [5] have reported that RETiNbO6 compounds with lanthanide of atomic numbers in the range 57–63 have orthorhombic aeschynite structure and those in the range 64–71 have orthorhombic euxenite structure. As per the report by Sebastian et al. [6] the aeschynite RETiNbO6 materials have positive Tf with high dielectric constant while euxenite compounds have negative Tf with a lower dielectric constant. Surendran et al. [7] have reported a similar observation in the case of RETiTaO6 ceramics also. It is possible to optimize the microwave dielectric properties of these ceramics by preparing suitable solid solutions and composites of aeschynite and euxenites. Solomon et al. [8] and Surendran et al. [9] reported that by making suitable solid solutions of both aeschynite and euxenite structures, it is possible to get compositions having low Tf and desirable microwave dielectric properties. Oishi et al. [10] have reported the microwave dielectric properties and crystal structure of Sm(Nb1−xTax)(Ti1−yZry)O6 ceramics. Recently Kumar et al. [11] and Joseph et al. [12] reported the effect of yttrium substitution for Nd on NdTiTaO6 ceramics and NdTiNbO6 ceramics and obtained near zero temperature coefficient of resonant frequency (Tf) along with good dielectric properties. Raman scattering studies to explain the structure and microwave dielectric properties of this type materials have also become significant in recent years [13–15]. Jacob et al. [16] reported the photoluminescence and dielectric properties of LnTiTaO6 (Ln=Ce, Pr and Sm) polycrystals. In the present work, we report the synthesis, structural analysis, microwave and the optical properties of Ln2/3Gd1/3TiNbO6 where Ln=Ce, Pr, Nd and Sm.
2 Experimental
The compositions Ln2/3Gd1/3TiNbO6, where Ln=Ce, Pr, Nd and Sm, abbreviated as CGTN, PGTN, NGTN and SGTN respectively, are prepared through the conventional solid-state route. The high purity oxides (>99%)of CeO2, Pr2O11,Nd2O3,Sm2O3,TiO2,Nb2O5 and Gd2O3 are used. Conventional solid state ceramic route is used to prepare polycrystalline samples. Samples are calcined at 1,250 °C, pellitized and sintered in the range 1,350–1,435 °C. The densities of well- polished samples are calculated using Archimedes method. Powdered samples are used for X-ray diffraction (Philips Expert Pro) studies using Cu–Kα radiation. The cell parameters are calculated using the least square method and the theoretical densities are determined. Polished sample is thermally etched at 1,300 °C and is used for Scanning Electron Microscopy (SEM) (JEOL JSM 5610 LV). The dielectric properties of the samples are measured in the microwave frequency region using the cavity resonator method with the help of network analyzer. The temperature coefficient of resonant frequency is also measured over a range of temperature 30–70 °C. The FT-Raman spectra of the compounds are recorded over the range 50–1,000/cm using a Bruker RFS 100/s FT Raman spectrometer using an Nd:YAG laser source lasing at 1,064 nm and a Ge diode detector. The resolution of the instrument is 4 cm−1 and the power level used is 25 mW. The photoluminescence spectra of the samples were also taken and the emission lines are identified. The absorption spectra of a sample were also recorded and the bandgap energy was estimated. The photoluminescence spectra are taken using Perkin Elmer LS 55 Xenon Lamp Spectrometer. The UV spectrum is taken using The Double beam UV–Vis Spectrometer and the bandgap is identified.
3 Results and discussion
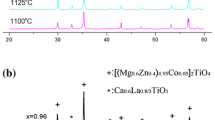
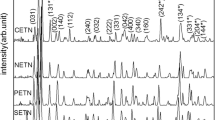
Figure 1a shows the XRD pattern of CGTN. It is a mixture of CeTiNbO6 (ICDD file 15-864), GdTiNbO6 (ICDD file 27-221), TiO2 (ICDD file 21-1276) and Nb2O5 (ICDD file 30-873). The reflections of CeTiNbO6 and GdTiNbO6 are indexed and the other reflections are marked as (*) in the pattern. Hence it is clear that CGTN does not form an exact solid solution and is nearly a composite.
Figure 1b shows the XRD patterns of PGTN, NGTN and SGTN ceramics. The reflections of these compounds are similar to that of aeschynite structure. That is exact solid solutions are formed in these compounds. The microwave dielectric properties, the lattice parameters and theoretical densities of Ln2/3Gd1/3TiNbO6 where Ln=Ce, Pr, Nd and Sm, are given in Table 1. As Ln moves from left to right in the periodic table, there is a gradual decrease in εr and Tf and a gradual increase in experimental (ρexp) and theoretical (ρthe) densities. εr varies from 54 to 45 and Tf from +67 to +50 for pure LnTiNbO6 [6, 12]. In the Gadolinium (Gd) substituted sample εr varies from 46 to 41.8 and Tf from +52 to +25. Therefore by the substitution of Gd, the Tf is reduced considerably with a slight decrease in dielectric constant.
Figures 2a and 3 shows the variation in εr and τf with respect to the average ionic radii of Ln and Gd. There is a gradual increase in εr and τf with the increase in average ionic radii. Figure 2b shows that the experimental density and theoretical density decrease with increase in the average ionic radii of lanthanides. These variations are in agreement with the earlier reports [9–11].
UV absorption spectra of the compound NGTN is given in Fig. 4. As neodymium is a lanthanide, it absorbs photons in the UV and visible regions. The transitions responsible for this absorption appear to involve the various energy levels of 4f electrons. These inner orbitals are largely screened from external influences by electrons occupying orbital with higher principal quantum numbers. As a consequence its spectra consist of narrow, well defined and characteristic peaks. Spectral characteristics of gadolinium involve electronic transitions among the various energy levels of 4d orbital. The absorption bands are often broad and strongly influenced by chemical environmental factors [17]. The material has absorption bands in the wavelength range 500–800 nm. Average particle size is calculated using Scherrer formula. A graph (Fig. 5) was plotted between (αhυ)2 and hυ (in eV), where α is the absorption coefficient. The value of the band gap is estimated by extrapolating the steep portion of the graph to zero absorbance. This tangential line coincides with the X axis at 2.9 eV and the corresponding wavelength is 417.6 Å.
Powdered samples are subjected to photoluminescence studies. Scanning was done in the range 400–700 nm and was excited at 370 nm. The spectra obtained are given in Fig. 6. The transitions are identified on the basis of the data book by Payling and Larkins [18]. The photoluminescence spectrum of CGTN shows emission lines at 423, 443, 460, 557 and 664 nm. The two characteristic peaks at wavelengths 423 and 443 nm are due to the transitions 9F 04 -5H4 and 9F 04 -7G4 of Gadolinium atom and that at 460 nm is due to the transition of 4F 45 -4F *4.5 of Niobium atom. The line at 557 nm is due to the transition 5I0 05 -A 05 of Cerium atom. The line at 664 nm is due to the transition of 3F2-5G 04 of Titanium atom.
The PL spectrum of PGTN shows emission lines at 422, 442, 497, 522 and 664 nm. The line observed at 422 nm is due to the transition 9p3-0A 04 due to Gadolinium atom and that observed at 442 nm is due to the transition 4I 015/2 -0A13/2 of Praseodymium. The line at 497 nm is due to the transition 4G 45 -4F 35 of Niobium. The lines at 422 and 664 nm are due to the transition 3F4-3F 04 and 3F2-5G 04 respectively of Titanium.
The PL spectrum of NGTN show emission lines at 442, 460, 485, 559 and 664 nm. The lines observed at 442 and 485 nm are due to transition 5I 06 -A 06 and 5I4-0A 03 due to Neodymium atom respectively and that at 460 nm is due to the transitions 7D 05 -7G5 of Gadolinium atoms. The line at 559 nm is due to the transition of 4D 25 -4D 15 of Niobium atom and that at 664 nm is due to the transition of 3F2-5G 04 of Titanium.
For SGTN the PL spectrum shows emission lines at 460, 485, 529, 664 and 696 nm. The emission lines observed at 484 nm are due to the transition 7F4-5F 04 of Samarium. The line at 460 nm is due to the transition 9F 05 -7H6 of Gadolinium. The line at 529 and 664 nm are due to the transitions of 5F2S-3P 02 and 3F2-5G 04 respectively of Titanium. The line observed at 696 nm is due to the transition 4G 25 -6D 25 of Niobium.
Figure 7 shows the Raman spectra of CGTN and SGTN and their spectral data and band assignments are given in Table 2. According to Qi et al. [5] RETiNbO6 with RE ions of atomic number in the range 57–63 have orthorhombic aeschynite structure and those in the range 64–71 have orthorhombic euxenite structure. Paschoal et al. [14] have reported the Raman scattering study of the microwave dielectric system RETiTaO6 for 15 different lanthanide of isovalance ions. The Raman spectra of CGTN and SGTN are found to be similar to that reported by Paschoal et al. for the aeschynite structure. The spectra are exactly similar to that reported for CeTiTaO6 and GdTiTaO6 both belonging to the aeschynite structure. The CGTN has most of the bands similar to that of aeschynite structure. However the bands in the Nb compounds have slightly lower intensity than that associated with Ta compounds due to the mass difference between the two. To conclude, both CGTN and SGTN have aeschynite orthorhombic structure.
4 Conclusion
Solid solutions of Ln2/3Gd1/3TiNbO6 (Ln=Ce, Pr, Nd and Sm) ceramics were prepared through the conventional solid state route. The materials were calcined at 1,250 °C and sintered at optimized temperature in the range 1,350–1,435 °C. The structure of the system was analyzed by XRD and vibrational spectroscopic studies. Cerium based composition had additional reflections other than that of aeschynite structure. For Pr, Nd and Sm based systems, solid solutions were formed. The sinterability and surface morphology were analyzed using SEM. Microwave dielectric properties were measured using the cavity resonator method. Thermal stability is achieved without much variation in dielectric constant of LnTiNbO6 ceramics. The UV spectra were studied and the bandgap energy is estimated. Photoluminescence spectra were analyzed and the corresponding transitions are identified.
References
M. Reaney, D. Iddles, J. Am. Ceram. Soc. 89, 2063 (2006)
W. Wersing, Curr. Opin. Solid. State Mater. Sci. 1, 715–731 (1996)
R.J. Cava, J. Mater. Chem. 11, 54–62 (2001)
I. Komkov, Dokl. Acad. Nauk 148, 1182 (1963)
X. Qi, R. Illingworth, H.G. Gallagher, T.P.J. Han, B. Henderson, Potential laser gain media with stoichiometric formula RETiNbO6. J. Cryst. Growth 160, 111–118 (1996)
M.T. Sebastian, S. Solomon, R. Ratheesh, J. George, P. Mohanan, J. Am. Ceram. Soc. 84(7), 1487–1489 (2001)
K.P. Surendran, P. Mohanan, M.T. Sebastian, J. Eur. Ceram. Soc. 23, 2489–2495 (2003)
S. Solomon, M. Kumar, K.P. Surendran, M.T. Sebastian, P. Mohanan, Mater. Chem. Phys. 67, 291 (2001)
K.P. Surendran, M.R. Varma, P. Mohanan, M.T. Sebastian, J. Am. Ceram. Soc. 86, 1695 (2003)
T. Oishi, A. Kan, H. Ohsato, H. Ogawa, J. Eur. Ceram Soc. 26, 2075–2079 (2006)
H.P. Kumar, J.K. Thomas, M.R. Varma, S. Solomon, J. Alloys. Compd. 455, 475 (2008)
S. Joseph, M.K. Suresh, J.K. Thomas, A. John, S. Solomon, Int. J. Appl. Ceram. Technol. 7, 129–134 (2009)
R. Ratheesh, H. Sreemoolanathan, S. Solomon, P.R. Ratheesh, H. Sreemoolanathan, M.T. Sebastian, Vibrational analysis of Ba5−xSrxNb4O15 dielectric ceramics. J. Solid State Chem. 131, 2–8 (1997)
C.W.A. Paschoal, R.L. Moreira, C. Fantini, M.A. Pimenta, K.P. Surendran, M.T. Sebastian, Raman scattering study of RETiTaO6 dielectric ceramics. J. Eur. Ceram. Soc. 23, 2661–2666 (2003)
M.-Y. Chen, C.-T. Chia, I.-N. Lin, L.-J. Lin, C.-W. Ahn, S. Nahm, Microwave properties of Ba(Mg1/3Ta2/3)O3, Ba(Mg1/3Nb2/3)O3 and Ba(Co1/3Nb2/3)O3 ceramics revealed by Raman scattering. J. Eur. Ceram. Soc. 26, 1965–1968 (2006)
L. Jacob, H. Padmakumar, K.G. Gopchandran, J.K. Thomas, S. Solomon, Photoluminescence and dielectric properties of LnTiTaO6 (Ln=Ce,Pr,Sm) polycrystals. J. Mater. Sci. Mater. Electron. 18(8), 831–835 (2007)
D.A. Skoog, F.J. Holler, T.A. Nieman, Principle of Instrumental Analysis, 5th edn. (Harcourt Asia Pte Ltd., Singapore, 1998), pp. 335–337, 355–358
R. Payling, P. Larkins (Wiley Verlag, New York, June 2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
John, A., Joseph, S., Manu, K.M. et al. Structure, microwave dielectric and optical properties of Ln2/3Gd1/3TiNbO6 (Ln=Ce, Pr, Nd and Sm) ceramics. J Mater Sci: Mater Electron 22, 776–780 (2011). https://doi.org/10.1007/s10854-010-0210-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-010-0210-z