Abstract
The microstructure and dielectric properties of x[(Mg0.6Zn0.4)0.95Co0.05]2TiO4–(1 − x)Ca0.6La0.8/3TiO3 (x = 0.9–0.96) ceramics prepared via conventional solid-state method were investigated using scanning electron microscopy and a network analyzer. Mixed phases of [(Mg0.6Zn0.4)0.95Co0.05]2TiO4 and Ca0.6La0.8/3TiO3 were detected using X-ray diffraction. The microwave dielectric properties are strongly correlated with the sintering temperature. Temperature coefficient of resonant frequency could be controlled by amount of Ca0.6La0.8/3TiO3 and thus near-zero temperature coefficient value can be obtained. With x = 0.94 sintered at 1175 °C for 4 h, the proposed dielectric material, applicable to microwave devices, has the following dielectric properties: a dielectric constant ~ 18.1, a quality factor value of ~ 156,000 (at 9 GHz), and temperature coefficient of resonant frequency of 1.2 ppm/°C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microwave dielectrics with high Q values at frequencies above 10 GHz are very important for advances in satellite communication and broadcasting systems. A number of such materials have thus been investigated and developed [1,2,3,4,5,6,7,8]. The ideal dielectric material should have a high dielectric constant (εr), a low dielectric loss (high Q × f value), and a near-zero temperature coefficient of resonant frequency (τ f ). These three parameters are correlated to the size, frequency selectivity, and temperature stability of the system, respectively. The high-quality factor (inverse of the dielectric loss, Q = 1/tanδ) plays a prominent role, as Q×f is almost constant in the microwave region.
MgTiO3 and Mg2TiO4, which belong to the MgO–TiO2 binary system, are good candidates for microwave applications. Among them, Mg2TiO4-based ceramics which possessed a spinel-type structure and a space group of Fd-3m (227) have attracted much attention and wide applications as dielectric resonators for millimeter-wave applications due to its high-performance microwave dielectric properties in the last decade [9,10,11,12,13,14]. Furthermore, partially replacing Mg by Zn and Co in Mg2TiO4, the [(Mg0.6Zn0.4)0.95Co0.05]2TiO4 (MZCT) ceramic sintered at 1250 °C possesses a εr of ~ 18, a high Q × f value of ~ 206,000 GHz (at 10.4 GHz) and a negativeτf value of − 20.8 ppm/°C [15]; however, the negative τf limits its temperature stability for practical applications. Thus, microwave ceramics with positive τf and appropriate high Q × f value are necessary to stoichiometrically mix with MZCT to achieve near-zero temperature coefficient of resonant frequency. Among them, Ca0.6La0.8/3TiO3 (CLT) is a very good candidate due to a high εr of ~ 100, a Q × f value higher than 20,000 GHz, and a τf value of + 212 ppm/°C [16,17,18]. In this work, xMZCT–(1 − x)CLT was investigated to systematically control the τf value of mixed ceramics. A comprehensive study of microwave dielectric properties and microstructures of xMZCT–(1 − x)CLT composite ceramics was carried out.
2 Experimental procedure
The starting materials were high-purity oxide powders (> 99.9%): MgO, ZnO, TiO2, CoO, CaO, and La2O3. The powders were separately prepared according to the desired stoichiometry of [(Mg0.6Zn0.4)0.95Co0.05]2TiO4 (MZCT) and Ca0.6La0.8/3TiO3 (CLT). They were then ground in distilled water for 12 h in a ball mill with agate balls. The prepared powders were dried and calcined at 925 and 1000 °C for 4 h in air. The calcined powders were mixed according to the molar fraction xMZCT–(1 − x)CLT and then remilled for 12 h. A fine powder with 3 wt% of a 10% solution of polyvinyl alcohol (PVA 500, Showa, Japan) used as a binder was pressed into pellets, 11 mm in diameter and 5 mm in thickness, under a pressure of 200 MPa. The pellets were sintered at temperatures of 1100–1225 °C for 4 h in air. The heating and cooling rates were both set at 10 °C/min.
The crystalline phases of the calcined powder and the sintered ceramics were identified using X-ray diffraction (XRD). The microstructure and sintered surfaces were observed using scanning electron microscopy (SEM; Philips XL-40FEG). Energy-dispersive X-ray spectroscopy (EDS) was used to identify the existence of secondary phases. The bulk densities of the sintered pellets were measured using the Archimedes method. The dielectric constant (εr) and the quality factor values (Q) at microwave frequencies were measured using the Hakki-Coleman [19] dielectric resonator method under TE011 and TE01σ modes as modified and improved by Courtney [20]. The dielectric resonator was positioned between two brass plates. The HP8757D network analyzer combined with HP8350B sweep oscillator was employed in the measurement. The same technique was applied in measuring the temperature coefficient of resonant frequency (τf). The test set was placed over a thermostat in the temperature range of + 25 to + 80 °C. The τf value (ppm/°C) was calculated by noting the change in resonant frequency (Δf).
where f1 and f2 represent the resonant frequencies at T1 and T2, respectively.
3 Results and discussion
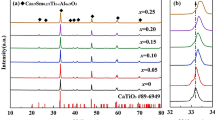
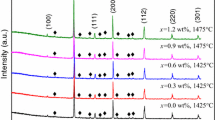
Figure 1a, b show the XRD patterns of the 0.94MZCT–0.06CLT ceramics sintered at various temperatures for 4 h and xMZCT–(1 − x)CLT ceramics sintered at 1175 °C for 4 h with x = 0.9–0.96, respectively. The notation of + or * above each significant peak represents phase formed in [(Mg0.6Zn0.4)0.95Co0.05]2TiO4 and Ca0.6La0.8/3TiO3, respectively. In all the XRD patterns, the spinel-type structure MZCT was the major phase and the pure orthorhombic structured CLT was the minor phase. The formation of mixed phases in the 0.94MZCT–0.06CLT and xMZCT–(1 − x)CLT ceramic systems were due to structural differences; MZCT–CLT could not be obtained in solid solution. No secondary phases formed in 0.94MZCT–0.06CLT after sintering at 1100–1225 °C and xMZCT–(1 − x)CLT with x = 0.9–0.96.
Microstructures of 0.94MZCT–0.06CLT ceramics sintered at various temperatures for 4 h and xMZCT–(1 − x)CLT ceramics sintered at 1175 °C for 4 h with x = 0.9–0.96 are shown in Fig. 2a–i. Sparse microstructures developed with pores and small grains for the specimens of 0.94MZCT–0.06CLT sintered at 1100 °C. Along with the reduction in porosity, the grain size increased continuously with increasing sintering temperature. For the specimen sintered at 1175 °C, pores were almost eliminated. The grain size grew rapidly at higher sintering temperatures, leading to a non-uniform grain morphology that increased the probability of pore formation. At 1200 and 1225 °C, porous microstructures which might degrade the microwave dielectric properties of the ceramics were observed due to inhomogeneous grain growth. In addition, microstructures of xMZCT–(1 − x)CLT ceramics with x = 0.9–0.94 shows increasing densification and then pores were observed with x = 0.96 due to overgrown grain.
The EDS spectrums of spot A and spot B marked in Fig. 2d were shown in Fig. 3, respectively. EDS analysis of 0.94MZCT–0.06CLT sintered at 1175 °C shows that the large grains (spot A) mainly contained Mg, Co, Ti, Zn, and O, and the small cubic grains (spot B) were mainly composed of Ca, La, Ti, and O. Table 1 shows approximate molar ratio for spot A and B in detail. This indicates that the large grains belonged to MZCT and the small cubic grains belonged to CLT, which is consistent with the XRD results and known higher sintered temperature for CLT.
EDS spectrum of 0.94MZCT–0.06CLT ceramics for spots a, b sintered at 1175 °C for 4 h. Spots a, b indicate large and small grains marked in Fig. 2d, respectively
The bulk density of the xMZCT–(1 − x)CLT ceramics sintered at various temperatures for 4 h are shown in Fig. 4. The bulk density increased with temperature to a maximum value at 1175 °C for all x composition, and thereafter decreased. The maximum value of 3.71 g/cm3 was obtained at x = 0.90 and 1175 °C, which shows the same maximum point as dielectric constant curves (Fig. 5). A density reduction due to abnormal grain growth was observed in Fig. 2e, f for the samples sintered at 1200 and 1225 °C.
The dielectric constant curves of the xMZCT–(1 − x)CLT ceramic at various sintering temperatures for 4 h are shown in Fig. 5. The dielectric constant slightly increased with increasing sintering temperature. The relationship between εr values and sintering temperature shows the same trend as that between density and sintering temperature since higher density means lower porosity. εr values of 0.94MZCT–0.06CLT ceramics increased from 16.51 to 18.1 when the sintering temperature was increased from 1100 to 1175 °C. A maximum εr value of 19 was obtained for the 0.9MZCT–0.1CLT ceramic sintered at 1175 °C for 4 h. After reaching a maximum at 1175 °C, it decreased, following the same trend as that of bulk density. The trend of εr was consistent with that of density.
Q × f is affected by extrinsic factors (e.g., secondary phases, density, and porosity) and intrinsic factors (e.g., crystal structure and lattice vibration modes). Here, the Q × f values increased from 1100 to 1175 °C and reached a maximum value at 1175 °C and thereafter slightly decreased as the sintering temperature of 1200–1225 °C under all x ratio, as shown in Fig. 6. This remarkable increase is explained by grain growth and the slight decrease may be attributed to abnormal grain growth and micro cracks. The maximum Q × f value was 195,600 GHz for the 0.96MZCT–0.04CLT ceramic sintered at 1175 °C.
Figure 7 shows the τf value as a function of x for xMZCT–(1 − x)CLT ceramics sintered at various temperatures for 4 h. The τf values of mixed materials are well known to be correlated to the secondary phase, additives, and material composition. Because CLT has a large positive τf value, the τf value of xMZCT–(1 − x)CLT ceramics rapidly increased from negative to positive value with increasing CLT content. 0.94MZCT–0.06CLT shows a near-zero τf, and furthermore τf value of xMZCT–(1 − x)CLT ceramic system would be controllable by adjusting the CLT content. The microwave dielectric properties of the xMZCT–(1 − x)CLT ceramic system sintered at 1175 °C for 4 h are summarized in Table 2. When the (1 − x) value was increased from 0.04 to 0.1, the τf value of the xMZCT–(1 − x)CLT ceramic ranged from − 10 to 10 ppm/°C. The 0.94MZCT–0.06CLT ceramic shows almost perfect temperature stability with τf = ~ 1.2 ppm/°C.
To further demonstrate high quality of this ceramic material system, band-pass filters using various dielectric substrates were simulated and discussed. The physical layout of the designed filter with a center frequency of 2.4 GHz is shown in Fig. 8, and their electromagnetic (EM) simulation results are listed in Table 3 in detail. Compared to FR4 and Al2O3, the filter using the substrate of 0.94MZCT–0.06CLT ceramic shows not only a tremendous reduction in insertion loss but also a large reduction in size. It is attributed to high performance microwave dielectric properties of 0.94MZCT–0.06CLT. This design approach enables one to use an EM simulator (IE3D) to complete the filter design to determine the physical dimensions of the filter.
4 Conclusion
In this paper, xMZCT–(1 − x)CLT (x = 0.9–0.96) ceramics were synthesized and studied. The spinel-type structure MZCT was the major phase and the pure orthorhombic structured CLT was the minor phase. From physical and electrical data, the microwave dielectric properties were strongly related to the density, secondary phase, sintering temperature, and microstructure of the samples. The τf values of this ceramics demonstrated controllable by adjusting the CLT content. At 1175 °C, the 0.94MZCT–0.06CLT ceramic demonstrated excellent microwave dielectric properties: εr of ~ 18.1, Q × f of ~ 156,000 GHz, and τf of ~ 1 ppm/°C. Band-pass filter using 0.94MZCT–0.06CLT as substrate confirmed advantages in insertion loss and layout size compared to FR4 and Al2O3. Thus, this dielectric ceramic is a promising candidate for applications in wireless communication systems.
References
S. Nishigaki, H. Kato, S. Yano, R. Kamimura, Am. Ceram. Soc. Bull. 66, 1405 (1987)
K. Wakino, K. Miani, H. Tamura, J. Am. Ceram. Soc. 67, 278 (1984)
T. Takada, S.F. Wang, S. Yoshikawa, S.J. Jang, R.E. Newnham, J. Am. Ceram. Soc. 77, 1909 (1994)
M.P. Seabra, V.M. Ferreira, Mater. Res. Bull. 37, 255 (2002)
K.H. Yoon, W.S. Kim, E.S. Kim, Mater. Sci. Eng. B 99, 122 (2003)
P.S. Anjana, I.N. Jawahar, M.T. Sebastian, J. Mater. Sci. Mater. Electron. 20, 587 (2009)
Y.C. Chen, C.F. Su, M.Z. Weng, H.M. You, K.C. Chang, J. Mater. Sci.: Mater. Electron. 25, 2120 (2014)
C.Y. Su, C.-L. Huang, W.-H. Lee, J. Ceram. Soc. Jpn. 116, 935 (2008)
C.L. Huang, C.H. Shen, J. Am. Ceram. Soc. 92, 384 (2009)
S.H. Lin, Y.B. Chen, Ceram. Int. 43, 296 (2017)
H.J. Cha, D.H. Kang, Y.S. Cho, Mat. Res. Bull. 42, 265 (2007)
J.J. Wang, J. Alloy. Compd. 486, 423 (2009)
C.H. Shen. C.L. Pan, S.H. Lin, Mater. Res. Bull. 169, 65 (2015)
S.S. Rajput, S. Keshri, J. Alloy. Compd. 581, 223 (2013)
S.H. Lin, Y.B. Chen, Ceram. Int. 43, 2368 (2017)
J.M. Li, T. Qiu, J. Mater. Sci.: Mater. Electron. 19, 245 (2014)
Y. Yan, Z. Li, M. Zhang, P. Sun, Y. Xu, Ceram. Int. 43, 8534 (2017)
Z. Yue, F. Zhao, Z. Gui, L. Li, J. Alloy. Compd. 395, 126 (2005)
B.W. Hakki, P.D. Coleman, IEEE Trans. Micro. Theory Tech. 8, 402 (1960)
W.E. Courtney, IEEE Trans. Microw. Theory Tech. 18, 476 (1970)
Acknowledgements
The authors would like to express their appreciation for the financial support for this study from the Ministry of Science and Technology, Taiwan, R.O.C., under Grant MOST 105-2221-E-029-004 and 106-2221-E-029-002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, YB., Tseng, ZL., Chen, LC. et al. Crystal structure and microwave dielectric properties of [(Mg0.6Zn0.4)0.95Co0.05]2TiO4-modified Ca0.6La0.8/3TiO3 cordierite ceramics with a near-zero temperature coefficient. J Mater Sci: Mater Electron 29, 10709–10714 (2018). https://doi.org/10.1007/s10854-018-9137-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9137-6