Abstract
In order to further enhance the properties of lead-free solder alloys such as SnAgCu, SnAg, SnCu and SnZn, trace amount of rare earths were selected by lots of researchers as alloys addition into these alloys. The enhancement include better wettability, physical properties, creep strength and tensile strength. For Sn3.8Ag0.7Cu bearing rare earths, when the rare earths were La and Ce, the creep-rupture life of solder joints can be remarkably improved, nine times more than that of the original Sn3.8Ag0.7Cu solder joints at room temperature. In addition, creep-rupture lifetime of RE-doped solders increases by over four times for SnAg and seven times for SnCu. This paper summarizes the effects of rare earths on the wettability, mechanical properties, physical behavior and microstructure of a series of lead-free solders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Increasing global concern about the environment is bringing regulatory (European directives) and consumer (“green products”) pressure on the electronics industry in Europe and Japan to reduce or completely eliminate the use of lead (Pb) in products [1]. Therefore, the development of lead-free solders has become an essential and urgent task in the electronics packaging industry. The choice of lead-free alloys is based on a comparison of solder properties and indicated that SnAgCu could be adopted as a general-purpose standard alloy. However, it is also found that SnAgBi type alloys are likely to be used for surface mount consumer products and that SnCu solders may be developed for using in wave soldering situation where the cost of the alloy is a particularly significant consideration [2]. Moreover, the eutectic Sn9Zn alloy appears to be an attractive one with a relatively low melting temperature of 198.5 °C [3], because the Zn element is quite active, SnZn system solders have a worse wetting and corrosion behavior [4]. If these problems can be overcome, they remain an attractive positions due to the closeness of their melting temperatures to that of SnPb. Therefore, it is expected that the addition of trace amount element will further improve these performance of lead-free solder alloys.
In the past investigation, adding minute amount of rare earth (RE) to series of lead-free alloys is considered to be an effective way to improve their properties. Rare earth (RE) elements have been called the “vitamin” of metals, which means that a small amount of RE elements can greatly enhance the properties of metals [5]. RE elements are the surface-active element, which plays an important role in metallurgy of materials, such as refinement of microstructure, alloying and purification of materials and metamorphosis of inclusions [6]. In this paper, we summarize the development in lead-free solder alloys with addition of RE elements and analyze the effect of RE elements on wettability, tensile strength, creep properties and microstructures of various lead-free alloys, such as SnAgCu, SnAg, SnCu, SnZn.
2 Wetting properties
Wettability is defined by the contact angle between the liquid drop and solid substrate, which is a direct consequence of the interface force equilibrium at the location where three phases meet [7, 8]. Wetting, however, very important for solders because reliable interconnection require good wetting, good wetting results in well-formed circuit-board solder fillets [9].The contact angles formed at the surface of the Cu substrate. If there are IMCs formed during the wetting reaction, the equilibrium contact angle is determined by the interfacial surface tension.
Owing to its better thermo-mechanical property and wettability, SnAgCu alloy has been drawn special atte ntion and regarded as the most promising lead-free substitutes for the conventional SnPb solder [10]. Comparative wetting angle of the four solders using an RMA flux is displayed in Fig. 1 [11] the wetting angle of Sn3.5Ag0.7Cu is lower than Sn2.5Ag0.7Cu. It is due to the lower melting point of Sn3.5Ag0.7Cu alloy. The addition 0.1% RE to Sn3.5Ag0.7Cu can significantly improve the wetting angle. Angle of Sn3.5Ag0.7Cu0.25RE is also lower, but higher than 0.1%RE addition.
Wetting angle of solder alloys [11]
Figure 2 shows the relationship between RE content and contact angle. It is found that RE addition to the SnAg alloy of up to 0.5 wt.% resulted in a decrease in the values of mean contact angle, a minimum contact angle of 31±2° was observed with 0.5 wt.% [12].
Influence of RE addition on the wettability in the SnAgRE solder[12]
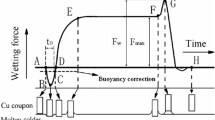
The Sn0.7Cu solder has been considered as a lead-free alternative to lead-tin alloys in waving soldering [13]. Various small amount of rare earth (RE) elements, which are mainly Ce and La, have been added to the SnCu alloy to form new solder alloy. The wetting force and angle of Sn0.7Cu solder are also improved with the addition of 0.5% RE elements. The wetting force and angle of Sn0.7Cu and Sn0.7Cu0.5RE are compared with that of Sn37Pb, as shown in Fig. 3 [14]. The traditional eutectic SnPb provides the highest wetting force, and Sn0.7Cu gives the lowest wetting force. The Sn0.7CuRE alloy has a smaller contact angle than that of Sn0.7Cu solder.
Variation of wetting force and angle of solders [14]
The eutectic SnZn alloy appears to be an attractive one with a relatively low melting temperature of 198.5 °C [15]. However, the wetting angle on copper substrate for SnZn solders is high with the flux used for SnPb solders. Figure 4 [16] shows the contact angle and wetting force of SnZnRE systems with the four different types of fluxes at 245 °C in the wetting balance test. In these figures, the RA flux is shown to provide sufficient wetting, whereas the RMA, R, and VOC-free fluxes could not provide wetting. It is confirmed by the observation that the molten solder has partly coated the surface of the test coupons, with some areas of the coupons uncoated. Wu [17] suggested that trace RE elements can decrease the surface tension of SnZn, with 0.05 and 0.0%RE addition, the wetting properties were greatly improved with the RA flux.
3 Melting temperature
Melting temperature is a crucial physical property and has important influence on the quality of solder alloys. In the great mass of melting temperature is a crucial physical property and has important influence on the quality of solder alloys. In the great mass of experiments, melting temperature is obtained by differential scanning calorimetry (DSC) measurement of enthalpy variation in melting process. The onset point of the DSC heating curve is related to the solidus temperature while the peak point is recognized as liquidus temperature of solder alloys.
By thermal method, it is found that the addition of trace rare earth Ce has a trifling effect on the melting temperature of Sn-3.0Ag-0.8Cu, the liquidus temperature is within 212–220 °C [18]. The DSC was employed to determine the effect of small amount of La (rare earth) on the melting characteristics of SnAgCu solder. Figure 5 shows the heat flow vs. temperature melting curves, on heating, for Sn3.9Ag0.7Cu and Sn3.9Ag0.7Cu0.5La. Both curves show a single melting peak (endothermic), with the onset temperature around 217 °C, indicating that La had little effect on the reflow temperature of the eutectic solder [19].
Heat flow vs. temperature curve for SnAgCu solder [19]
It had been demonstrated that the incorporation of a small amount (0.5–2 wt.%) of lutetium (rare earth) metal into lead-free solders makes the direct bonding of the solders to SiO2 possible, thermal analysis indicates that rare earth metal addition do not significantly alter the melting point, but maintain a melting point near 221 °C [20]. When the rare earths are La and Ce, there is no change of the liquidus temperature after the addition of RE elements into Sn3.5Ag system [14]. The same phenomena can be found for Sn0.7Cu and Sn9Zn alloys with the addition of rare earth elements.
4 Tensile properties of soldered joints
The tensile test method was employed to measure the mechanical properties of lead-free solder alloys. Dog bone-shaped bulk solder specimens for the uniaxial tensile were machined from solder bars. The ultimate tensile strength (UTS) is the maximum engineering stress which a material can understand in tension [5].
Tensile tests results of SnAgCu-XRE are shown in Fig. 6 [11]. With the increase of Ag and addition of rare earth elements, the tensile strength is improved. With the addition of trace rare earth elements the coarse β-Sn grains were refined, Cu6Sn5 and Ag3Sn intermetallics were finer according to the adsorption affection of the active rare earth elements, which are the reasons for the improvement of tensile strength of SnAgCu alloy with the addition of rare earth elements.
Tensile strength SnAgCu-XRE soldered joints [11]
Table 1 indicates the ultimate tensile strengths and elongations values of the alloys. Tensile strengths of the three solders can be seen to be similar except a lower elongation of Sn2.9Ag1.2Cu0.05RE compared with the other alloys. The tensile and shear strengths of solder joints were decline in the order Sn3.8Ag0.7Cu0.05RE, Sn2.9Ag1.2Cu0.05RE, to Sn3Ag0.5Cu0.05RE [21].
Figure 7 shows the results of tensile strength of SnCu-XRE, SnAg-XRE, SnZn-XRE lead-free solders [5, 11]. From the figure, it is found that all the tensile strength of lead-free solders are enhanced. However, if the amount of rare earth elements added is increased, the elongation to failure and tensile strength of lead-free solders decreases significantly [5, 22]. This may be due to the increase in the quantity of the hard RE-bearing intermetallics in the solders.
Tensile strength of various Pb-free alloys under constant strain rate [5]
5 Creep behavior
Low cycle fatigue, resulting from differences in coefficient of thermal expansion between components connected by solder joints, is a prime cause of joint failure in electronic industry [23, 24]. In these fatigue processes, however, creep mechanisms play an important role because of the high homologous temperatures involved. In addition, due to the low melting point of lead-free solders, creep deformation was dramatic since room temperature exceeded half of the solder’s melting point [25, 26].
The SnAg-based lead-free solder provides better mechanical properties, creep resistance, thermochanical fatigue behavior, and solderability on copper and copper alloys than the traditional Sn-Pb solder [27–29].
The effect of adding trace amount of rare earth on creep-rupture life of soldered joints is shown in Fig. 8. Figure 8a indicates that rare earth Ce can prolong the creep-rupture life of Sn3.0Ag0.28Cu soldered joints at room temperature markedly [18]. When the content of rare earth Ce is 0.1%, the creep-rupture life is the longest, up to nine times more than original alloys. Figure 8b shows the effect of misch rare earth on creep-rupture life of Sn2.5Ag0.7Cu soldered joints [30]. Trace amount of rare earth elements can make the structure homogenous and fine, and decrease the intermetallic compound layer thickness, due to rare earth elements processes a high affinity to Sn in the matrix [31–35]. So the creep-rupture life is enhanced significantly.
In the SnAg solder joint with addition of rare earth, the refined grains and particles decrease the distance for dislocations to pile up, making the solders more creep resistance, and producing a longer creep-rupture life and higher strength in the tension with less elongation. Extensive creep tests La-doped SnAg alloys were conducted by Pei M [36, 37]. It is found that doping increases the creep resistance of SnAg solders by as much as 15%, higher La doping tends to improve creep resistance more. In addition, La doping enhances the fatigue life by approximately five times.
For the low melting point of lead-free solders, such as Sn0.7Cu alloy, the creep behavior is more important because these alloys more likely be used at a homologous temperature at which creep would occur. As shown in Fig. 9 [38], the Sn0.7Cu0.5RE solder has much higher creep resistance than Sn0.7Cu. Additionally, the creep lifetime is longer in the case of Sn0.7Ag0.5Cu than Sn0.7Cu. It is well known that the decrease in grain size reduces the stress concentrations as grain boundaries, and therefore retards cavity nucleation, thus, the fine grain size β-Sn and more uniform dispersion of the intermetallic particles were the main reason of the improvement of creep resistance when doped with 0.5% RE elements [39].
Comparison of creep resistance between Sn0.7Cu and Sn0.7Cu0.5RE alloys [38]
From the melting temperature point-of-view, the eutectic Sn9Zn alloy is one of the best alternatives to traditional eutectic SnPb with a melting temperature of 199 °C, as compared with 183 °C of SnPb [40]. To make a comparison of the creep behavior of Sn9Zn with Sn40Pb all test were done with the same homologous temperatures and homologous stresses. As a result, the Sn9Zn shows a significantly higher creep resistance which could be correlated with the higher stress exponent and higher activation energy (n = 3.6; Q = 1.68 eV) in comparison to the Sn40Pb ally [41]. In the SnZnRE system, due to the addition of rare earth elements, the refined microstructure provided better mechanical properties, such as creep resistance, than original SnZn alloys [14].
6 Microstructures
The rare earth elements were found to have a significant effect on the microstructure of lead-free solder joints. Trace Y (rare earth) addition had little influence on the melting behavior, but the solder showed better wettability and mechanical properties, as well as finer microstructures, than found in original Sn3.8Ag0.7Cu solders [42]. With the addition of Y element, the thickness of intermetallics layer at the solder/Cu substrate interface was decreased in soldering and the growth of intermetallics was constrained after high temperature aging [43].
The typical microstructure shown in Fig. 10 [44] of chill cast Sn3.5Ag0.7Cu alloy, mainly consisted of phases of β-Sn, Cu6Sn5 and Ag3Sn. Because of the fast cooling rate provided by the Cu atom, the β-Sn phases were distributed in the ternary eutectic region. The grain size of β-Sn phases was about 10–20 μm. In some regions, small Ag3Sn intermetallics coexisted with the β-Sn phases. The formation of the microstructure was due to the solidification sequence of the ternary alloys. The addition of RE decreased the original β-Sn grain size to 5–10 μm, as shown in Fig. 10b [44]. The RE-bearing phase could not be detected due to the small amount of RE addition and its fine dispersion.
Microstructure of lead-free solder joints [44]. a Sn3.5Ag0.7Cu; b Sn3.5Ag0.7Cu0.25RE
For the addition of rare earth Ce into SnAgCu alloy, with the content of Ce increasing, the new black phase increase evidently, and the shape of RE phase takes on a block-like, snow-like or fishbone-like as shown in Fig. 11a, the microstructure is composed of a Sn-rich phase, rod-like Cu6Sn5 and grain Ag3Sn by SEM and EDX analysis [18]. When the rare earths were La and Ce, the RE phase in snowflake shape can be seen in Fig. 11b, which is assumed to aggregate at the inter-dendrites boundaries, was not found at the SnAgCuRE bulk solder, the reason why the web structure is not observed in the bulk solder is that the sizes of the bulk solder and joint are different, and the conditions of cooling rates differ also [45].
In the La-containing SnAgCu alloys, the LaSn3 intermetallics had a characteristic faceted geometry also referred to as “Chinese scripts”, which have also been observed in other systems [46–48]. Figure 12 shows the microstructure of a furnace-cooled Sn3.9Ag0.7Cu0.5La alloy, in which the LaSn3 intermetallics are approximately 75 μm in size [19].
The dendritic structural LaSn3 intermetallics [19]
The electronics industry is exploring the use of SnAg solders for electronic packaging applications for two reasons [49–52]. First, the solders contain no lead, so it is candidates for replacement of Pb-bearing solders which in the near future may be regulated or taxed. Second, the solders meet the increasing service and manufacturing requirements for varying melting temperature ranges. Small addition of Lu (0.5–2 wt.%) added to eutectic SnAg solder render it directly solderable to a silicon oxide surface. Figure 13 shows a cross sectional microstructure of the joint between the SnAgLu rare earth containing solder and the silica substrate, extensive EDX analyzes indicate that within the resolution of EDX, the rare earth Lu is essentially non-detectable in the faceted islands (marked A in Fig. 13) with an average size of 5 μm [20]. These islands have been identified as the intermetallic compound with a composition close to Sn3Lu [53].
SEM cross sectional micrograph showing the joint between the SnAgLu solder and silica. Lutetium is present in rectangular-shaped island marked as A [20]
The snowflakes were also found in the SnAg solder joint as shown in Fig. 14 [36, 37]. EDX studies conformed that there was indeed a very high concentration of La in the snowflake structures, it was also observed that the amount of LaSn3 IMC is dependent on the amount of La doping. With less La doping, the snowflakes are usually small and isolated; with higher La doping levels, larger snowflakes can be seen in cluster form, as seen in Fig. 14 for 0.25% La doping. Rare earth elements are well recognized as surface-active agents and previous researches have found that RE doping can dramatically reduce IMCs and their growth on solder/pad interfaces and also refine the microstructure of the alloy which results in improved mechanical properties of the solder [54]. Figure 15.
Lanthanum aggregation cluster in 0.25% La solder [36]
SEM micrographs for Sn0.7Cu and Sn0.7Cu0.5RE alloys [38]
SnCu solder assembly can be used to fabricate a reliable printed circuit assembly for consumer products, and can be accomplished using present SnPb assembly facility [55]. So some researchers and consortia recommend SnCu for lead-free wave soldering [56, 57]. Due to dissolution of Cu pad rapidly into liquid solder at the onset of the reflow process, it is between SnCu solders and Cu pads that the interfacial reactions take place, which is similar to the prior cases [58–60]. In the SnCu system, the Cu–Sn intermetallics were found to be the primary precipitates in the β-Sn matrix. The SEM micrograph of the Sn0.7Cu and Sn0.7Cu0.5RE are shown in Fig. 2 [38]. The Cu6Sn5 intermetallics can be seen in Fig. 2a, by adding the RE elements, the Cu6Sn5 flakes decrease in size and became finely dispersed in the eutectic network band, when comparing the microstructures of the Sn0.7Cu solders, since the solubility of the rare earth elements in the β-Sn matrix is rather low, the Sn–RE intermetallics are likely to be formed and dispersed at the eutectic band only [14, 38]. Additionally, in order to further improve the properties and microstructures of SnCu alloys, other element with rare earth elements together, such as Ni, were added into the SnCu solders by researchers [7, 61–65].
For SnZn solder alloy, mechanical properties [66], solderability [67] and oxidation behavior [68, 69] are investigated by many researchers, the general conclusion is despite their better mechanical properties, the SnZn alloys have poor wettability to the Cu substrate, which is generally related to the higher surface tension and oxidation sensitivity of Zn [70, 71]. Rare earth elements with concentrations of 0.05 and 0.1 wt.% primarily Ce and La were added to the SnZn eutectic alloy to improve the mechanical properties and refine the microstructure of original alloy [14, 16]. In the eutectic SnZn solder, the microstructure under backscattered scanning electron (BSE) microscopy examination is found to consist of the bright β-Sn matrix surrounded by the long plate-like dark Zn-rich phase as shown in Fig. 16 [14], with addition of 0.5 wt.% RE, two RE-bearing phases appear in the as-reflowed SnZn solder, namely 75Sn14Ce11La and 24Sn35Zn23Ce18La.
BSE micrographs of bulk microstructure of as-reflowed. a Sn-9Zn; b Sn-Zn-0.5RE [14]
7 Conclusion
Due to the implementation of the legislations to restrict the use of environmental unfriendly materials, the use of lead in electronic products is banned. SnAgCu, SnAg, SnCu, SnZn solders have emerged among various lead-free candidates as the most promising solder alloys to be utilized in microelectronic industries. However, with the advance of integrated circuit technology and the requirement of high density for high-speed circuitry, further research is required to assess in-service durability for lead-free solder alloys. Rare earth elements have been regards as the vitamin of metals, which means that a minute amount of RE elements may greatly enhance the properties of metals. In addition, compared to other elements, rare earth elements are so cheap. Therefore, adding minute amount of rare earth to SnAgCu solders is considered to be an effective way to improve the properties and microstructures of alloys, but rare earth elements has a trifling effect on the melting temperature of lead-free solders. Thus, the potential problems that would arise during production are required to be studied, such as long-term reliability and soldering processes. So much effort should be made for assessing in-service durability for lead-free solder joints with rare earth alloying addition. The results of these previews provide an important basis of understanding the development of lead-free solders with addition of rare earth elements.
References
B. Vandevelde, M. Gonzalez, P. Limaye, P. Ratchev, E. Beyne, Thermal cycling reliability of SnAgCu and SnPb solder joints: a comparison for several IC-packages. Microelectron. Reliab. 47(2–3), 259–265 (2007). doi:10.1016/j.microrel.2006.09.034
B. Richards, K. Nimmo, An analysis of the current status of lead-free soldering. British Department of Trade in Industry Report 1999, pp. 1–9
P.K. Shiue, L.W. Tsay, C.L. Lin, J.L. Ou, The reliability study of selected Sn–Zn based lead-free solders on Au/Ni-P/Cu substrate. Microelectron. Reliab. 43(3), 453–463 (2003). doi:10.1016/S0026-2714(02)00259-7
D.Q. Yu, H.P. Xie, L. Wang, Investigation of interfacial microstructure and wetting property of newly developed Sn–Zn–Cu solders with Cu substrate. J. Alloy. Comp. 385(1–2), 119–125 (2004). doi:10.1016/j.jallcom.2004.04.129
C.M.L. Wu, D.Q. Yu, C.M.T. Law, L. Wang, Properties of lead-free solder alloys with rare earth element additions. Mater. Sci. Eng. Rep. 44(1), 1–44 (2004). doi:10.1016/j.mser.2004.01.001
Y.W. Shi, J. Tian, H. Hao, Z.D. Xai, Y.P. Lei, F. Guo, Effect of small amount addition of rare earth Er on microstructure and property of SnAgCu solder. J. Alloy. Comp. 453(1–2), 180–184 (2008). doi:10.1016/j.jallcom.2006.11.165
B. Lu, J.H. Wang, H. Li, H.W. Zhu, X.H. Jiao, Effect of cerium on microstructure and properties of Sn-0.7Cu-0.5Ni. J. Chin. Rare. Earth. 25(2), 217–222 (2007)
B. Lu, H. Li, J.H. Wang, Y.H. Zhang, Influence of trace rare earth elements on the property of Sn–Ag–Cu lead-free solder. Rare. Met. Cemented. Carbides 35(1), 27–30 (2007)
K. Sugantuma, Advances in lead-free electronics soldering. Curr. Opin. Solid State Mater. Sci. 5(1), 55–64 (2001). doi:10.1016/S1359-0286(00)00036-X
L. Zhang, S.B. Xue, Y. Chen, Z.J. Han, J.X. Wang, S.L. Yu, F.Y. Lu, Effects of cerium on Sn–Ag–Cu alloys based on finite element simulation and experiments. J. Rare. Earths 27(1), 138–144 (2009). doi:10.1016/s1002-0721(08)60208-z
D.Q. Yu, J. Zhao, L. Wang, Improvement on the microstructure stability, mechanical and wetting properties of Sn–Ag–Cu lead-free solder with the addition of rare earth elements. J. Alloy. Comp. 376(1–2), 170–175 (2004). doi:10.1016/j.jallcom.2004.01.012
L. Wang, D.Q. Yu, J. Zhao, M.L. Hhuang, Improvement of wettability and tensile property in Sn–Ag–RE lead-free solder alloy. Mater. Lett. 56(6), 1039–1042 (2002). doi:10.1016/S0167-577X(02)00672-9
S.B. Xue, L. Zhang, L.L. Gao, S.L. Yu, H. Zhu, Current Situation and Prospect on Lead-free Solders affected with micro alloying elements. Welding & Joining. 2009 (3), 24–33
C.M. Law, Reliability and interfacial reaction of lead-free solder alloys doped with rare earth elements (City University of Hong Kong, Hong Kong, 2004)
X.Q. Wei, H.Z. Huang, L. Zhou, M. Zhang, X. Liu, On the advantages of using a hypoeutectic Sn–Zn as lead-free solder materials. Mater. Lett. 61(3), 655–658 (2007). doi:10.1016/j.matlet.2006.05.029
C.M.L. Wu, C.M.T. Law, D.Q. Yu, L. Wang, The wettability and microstructure of Sn–Zn–RE alloys. J. Electron. Mater. 32(2), 63–69 (2003). doi:10.1007/s11664-003-0238-4
C.M.L. Wu, D.Q. Yu, C.M.T. Law, L. Wang, The properties of Sn-9Zn lead-free solder alloys doped with trace rare earth elements. J. Electron. Mater. 31(9), 921–927 (2002). doi:10.1007/s11664-002-0184-6
X.Y. Zhao, M.Q. Zhao, X.Q. Cui, M.X. Tong, Effect of cerium on microstructure and mechanical properties of Sn–Ag–Cu system lead-free solder alloys. Trans. Nonferrous Met. Soc. China 17(4), 805–810 (2007). doi:10.1016/S1003-6326(07)60178-2
M.A. Dudek, R.S. Sidhu, N. Chawla, M. Renavikar, Microstructure and mechanical behavior of novel rare earth-containing Pb-free solders. J. Electron. Mater. 35(12S), 2088–2097 (2006). doi:10.1007/s11664-006-0318-3
A.G. Ramirez, H. Mavoori, S. Jin, Bonding nature of rare-earth-containing lead-free solders. Appl. Phys. Lett. 80(3), 338–340 (2002). doi:10.1063/1.1435075
W.M. Xiao, Y.W. Shi, Y.P. Lei, Z.D. Xai, F. Guo, Comparative study of microstructures and properties of three valuable SnAgCuRE lead-free solder alloys. J. Electron. Mater. 35(5), 1095–1103 (2006). doi:10.1007/BF02692572
S.B. Xue, L. Liu, Y.F. Dai, L.H. Yao, Effects of rare-earth element Ce on physical properties and mechanical properties of Sn–Ag–Cu lead-free solder. Trans. China Weld. Institution. 26(10), 23–26 (2005)
A.R. Geranmayeh, R. Mahmudi, Power law indentation creep of Sn-5%Sb solder alloy. J. Mater. Sci. 40(13), 3361–3366 (2005). doi:10.1007/s10853-005-0421-5
R. Mahmudi, A.R. Geranmayen, M. Bakherad, M. Allami, Indentation creep study of lead-free Sn-5%Sb solder alloy. Mater. Sci. Eng. A 457(1–2), 173–179 (2007). doi:10.1016/j.msea.2007.01.060
L. Zhang, S.B. Xue, Z.J. Han, J.X. Wang, L.L. Gao, Z. Sheng, Mechanical properties of fine pitch device soldered joints based on creep model. Chin. J. Mech. Eng. 21(6), 82–85 (2008). doi:10.3901/CJME.2008.06.082
L. Zhang, S.B. Xue, G. Zeng, Z.J. Han, S.L. Yu, Study on mechanical properties and numerical simulation of fine pitch devices soldered joints. Trans. China Weld. Institution. 29(10), 35–39 (2008)
C.M. Chuang, K.L. Lin, Effect of microelements addition on the interfacial reaction between Sn–Ag–Cu solders and the Cu substrate. J. Electron. Mater. 32(12S), 1426–1431 (2003). doi:10.1007/s11664-003-0111-5
M. Gonzalez, B. Vandevelde, E. Beyne, Thermo-mechanical analysis of a chip scale package (CSP) using lead-free and lead containing solder materials// European Microelectronics and Packaging Symposium, Prague, Czech Republic, June 2004, pp. 247–252
L. Zhang, S.B. Xue, Z.J. Han, S.L. Yu, Z. Sheng, The investigation of mechanical fracture morphology of lead-free soldered joints of fine pitch devices. Trans. China Weld. Institution. 29(9), 40–43 (2008)
K.K. Zhang, J. Yang, Y.L. Wang, Y.L. Fan, X. Zhang, Y.F. Yan, Research on creep properties of SnAgCuRE lead-free soldered joints//International Conference on Electronic Packaging Technology, Shanghai, China, 1–3 August, 2006
Y. Chen, S.B. Xue, X.C. Lv, Y.P. Liao, Influence of cerium on microstructure of Sn–Ag–Cu lead-free solder. Trans. China Weld. Institution. 26(10), 69–72 (2005)
X. Ma, F.J. Wang, Y.Y. Qian, F. Yoshida, Development of Cu–Sn intermetallic compound at Pb-free solder/Cu joint interface. Mater. Lett. 57(22–23), 3361–3365 (2003). doi:10.1016/S0167-577X(03)00075-2
Q. Liu, B. Lu, H. Li, J.H. Wang, H.W. Zhu, X.H. Jiao, Effect of adding 0.1% Ce into Sn-3.0Ag-0.5Cu solder alloy on its microstructure and intermetallic compounds with Cu substrate. J. Chin. Rare Earth Soc. 25(6), 707–712 (2007)
S.B. Xue, Y. Chen, X.C. Lv, Thermodynamic calculation and evaluation for Sn–Ag–Cu–Ce lead-free solder alloy system Sn–Ag–Cu–Ce. Trans. China. Weld. Institution. 26(5), 20–22 (2005)
X. Ma, Y.Y. Qian, F. Yoshida, Effect of La on the Cu–Sn intermetallic compound (IMC) growth and solder joint reliability. J. Alloy. Comp. 334(1), 224–227 (2002). doi:10.1016/S0925-8388(01)01747-9
M. Pei, J.M. Qu, Effect of Lanthanum doping on the microstructure of tin-silver solder alloys. J. Electron. Mater. 37(3), 331–338 (2008). doi:10.1007/s11664-007-0335-x
M. Pei, J.M. Qu, Creep and fatigue behavior of SnAg solders with Lanthanum doping. IEEE Trans. Compon. Packag. Tech. 31(3), 712–718 (2008). doi:10.1109/TCAPT.2008.922002
C.M.L. Wu, D.Q. Yu, C.M.T. Law, L. Wang, Microstructure and mechanical properties of new lead-free Sn–Cu–RE solder alloys. J. Electron. Mater. 31(9), 928–932 (2002). doi:10.1007/s11664-002-0185-5
C.M.L. Wu, A promising lead-free material for flip-chip bumps: Sn–Cu–RE//The Fourth international Conference on Advanced Semiconductor Devices and Microsystem, Smolenice Castle, Slovakia, October, 2002:17–26
H. Wang, S.B. Xue, Z.J. Han, J.X. Wang, Research status and prospect of Sn–Zn based lead-free solders. Welding & Joining. 2007 (2), 31–35
J. Villain, W. Jillek, E. Schmitt, T. Qasim, Properties and reliability of SnZn-based lead-free solder alloys//International IEEE Conference Asian Green Electronics, Hong Kong, China, January, 2004:38–41
H. Hao, J. Tian, Y.W. Shi, Y.P. Lei, Z.D. Xia, Studies on microstructure and performance of SnAgCuY lead-free solders doped with rare earth Y. Rare. Met. Mater. Eng. 35(S2), 121–123 (2006)
H. Hao, J. Tian, Y.W. Shi, Y.P. Lei, Z.D. Xia, Properties of Sn3.8Ag0.7Cu solder alloy with trace rare earth element Y additions. J. Electron. Mater. 36(7), 766–774 (2007). doi:10.1007/s11664-007-0138-0
C.M.L. Law, C.M.L. Wu, D.Q. Yu, L. Wang, J.K.L. Lai, Microstructure, solderability, and growth of intermetallic compounds of Sn–Ag–Cu–RE lead-free solder alloys. J. Electron. Mater. 35(1), 89–93 (2006). doi:10.1007/s11664-006-0189-7
Z.G. CHEN, Y.W. SHI, Z.D. XIA et al., Study on the microstructure of a novel lead-free solder alloy SnAgCu-RE and its soldered joints. J. Electron. Mater. 31(10), 1122–1128 (2002). doi:10.1007/s11664-002-0052-4
M.A. Dudek, R.S. Sidhu, N. Chawla, Novel rare-earth-containing lead-free solders with enhanced ductility. J. Miner. Met. Mater. Soc. 58(6), 57–62 (2006). doi:10.1007/s11837-006-0184-8
M.A. Dudek, N. Chawla, Three-dimensional (3D) microstructure visualization of LaSn3 intermetallic in a novel Sn-rich rare-earth-containing solder. Mater. Charact. 59(9), 1364–1368 (2008). doi:10.1016/j.matchar.2007.10.008
K.W. Moon, W.J. Boettinger, U.R. Kattner, F.S. Biancaniello, C.A. Handwerker, Experimental and thermodynamic assessment of Sn–Ag–Cu solder alloys. J. Electron. Mater. 29(10), 1122–1136 (2000). doi:10.1007/s11664-000-0003-x
C.H. Raeder, G.D. Schmeelk, D. Mitlin, T. Barbieri, W. Yang, L.F. Felton, R.W. Messler, D.B. Knorr, D. Lee, Isothermal creep of eutectic SnBi and SnAg solder and solder joints//Sixteenth International Electronics Manufacturing Technology Symposium, La Jolla, CA, USA, September 1994, pp. 1–6
D.H. Kim, Reliability study of SnPb and SnAg solder joints in PBGA packages (The University of Texas, Austin, 2007)
H. Johann, C. Hoyler, M. Schneegans, H. Torwesten, Evaluation of lead-free SnAg solder ball deposition and reflow processes for flip chip applications. Mircoelectronic Eng. 82(3–4), 581–586 (2005). doi:10.1016/j.mee.2005.07.072
L. Zhang, S.B. Xue, F.Y. Lu, Z.J. Han, J.X. Wang, Numerical simulation of soldered joints and reliability analysis of PLCC components with J-shape leads. China Weld. 17(2), 37–41 (2008)
H. Mavoori, A.G. Ramirez, S. Jin, Universal solders for direct and powerful bonding on semiconductors, diamond, and optical materials. Appl. Phys. Lett. 78(19), 2976–2978 (2001). doi:10.1063/1.1370985
M. Pei, Effects of lanthanum doping on the microstructure and mechanical behavior of a SnAg alloy (Georgia Institute of Technology, Georgia, 2007)
B. Trumble, Printed circuit assembly with no lead solder assembly process//Proceeding of the IEEE International Symposium on Electronics and the Environment, San Francisco, CA, USA,1997:25–27
D.J. Luo, X.Y. Lin, R.H. Liu, Choice of lead-free solder and its countermeasure. Electron. Process. Technol. 25(5), 202–204 (2004)
V. Sunappan, P. Collier, Lead-free wave soldering development for PCB assembly//Proceeding of the 53rd Electronic Components and Technology Conference, New Orleans, May, 2003:1829–1838
Y.Q. Zheng, Effect of surface finishes and intermetallics on the reliability of SnAgCu interconnects (University of Maryland, Maryland, 2005)
B. Lu, J.H. Wang, H. Li, H.W. Zhu, X.H. Jiao, Effect of 0.10% Ce on intermetallic compounds at Sn-0.7Cu-0.5Ni/Cu. Chin. J. Nonferrous. Met. 17(3), 390–395 (2007)
J.W. Jang, I.A.P.D. Silva, T.Y. Lee, J.K. Lin, D.R. Frear, Direct correction between microstructure and mechanical tensile properties in Pb-free solders and eutectic SnPb solder for flip chip technology. Appl. Phys. Lett. 482(23), 49–57 (2001)
S.B. Xue, J.X. Wang, S.L. Yu, Y.P. Shi, Z.J. Han, Effects of thermal cycling on mechanical property of chip resistor joints soldered with Sn–Cu–Ni–Ce solder. Trans. China Weld. Institution 29(4), 5–8 (2008)
Y.P. Shi, S.B. Xue, J.X. Wang, L.Y. Gu, W.H. Gu, Effects of Ce on spreadability of Sn–Cu–Ni lead-free solder and mechanical properties of soldered joints. Trans. China Weld. Institution. 28(11), 73–77 (2007)
J.X. Wang, S.B. Xue, Z.J. Han, Y.P. Shi, L. Zhang, Effects of Ce on physical properties and spreadability of Sn–Cu–Ni solder. Electron. Weld. Mach. 38(9), 42–45 (2008)
Z.J. Han, S.B. Xue, J.X. Wang, S.B. Wang, Effects of Sn–Cu–Ni–Ce solder on mechanical properties of micro-joints soldered with diode-laser soldering system. Trans. China Weld. Institution. 28(1), 33–36 (2007)
J.X. Wang, S.B. Xue, X. Huang, Z.J. Han, S.L. Yu, Effects of N2 protection on wettability of Sn–Cu–Ni–Ce lead-free solder. Trans. China Weld. Institution. 28(1), 49–52 (2007)
R.A. Islam, B.Y. Wu, M.O. Alam, Y.C. Chan, W. Jillek, Investigations on microhardness of Sn–Zn based lead-free solder alloys as replacement of Sn–Pb. J. Alloy. Comp. 392(1–2), 149–158 (2005). doi:10.1016/j.jallcom.2004.08.079
M.N. Islam, Y.C. Chan, M.J. Rizvi, W. Jillek, Investigations of interfacial reactions of Sn–Zn based and Sn–Ag–Cu lead-free solder alloys as replacement for Sn–Pb solder. J. Alloy. Comp. 400(1–2), 136–144 (2005). doi:10.1016/j.jallcom.2005.03.053
J.E. Lee, K.S. Kim, M. Inoue, J.X. Jiang, K. Suganuma, Effects of Ag and Cu addition on microstructural properties and oxidation resistance of Sn–Zn eutectic alloy. J. Alloy. Comp. 454(1–2), 310–320 (2008). doi:10.1016/j.jallcom.2006.12.037
H. Wang, S.B. Xue, W.X. Chen, Effects of Al addition on corrosion resistance and high-temperature oxidation resistance of Sn-9Zn lead-free solder. Electron. Weld. Mach. 38(9), 61–64 (2008)
R. Mahmudi, A.R. Geranmayeh, H. Noori, M. Shahabi, Impression creep of hypoeutectic Sn–Zn lead-free solder alloys. Mater. Sci. Eng. A. 491(1–2), 110–116 (2008). doi:10.1016/j.msea.2008.01.051
C.M.L. Wu, Y.W. Wong, Rare-earth additions to lead-free electronic solders. J. Mater. Sci.: Mater. Electron. 18(1–3), 77–91 (2007). doi:10.1007/s10854-006-9022-6
Acknowledgments
The authors greatly appreciate the financial support from the Nanjing University of Aeronautics and Astronautics Undergraduate Scientific Research Innovative(20080110), Six Kind Skilled Personnel Project of Jiangsu Province (CX07B_087z), and the Jiangsu General Colleges and Universities Postgraduate Scientific Research Innovative Plan(CX07B_087z).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Xue, Sb., Gao, Ll. et al. Effects of rare earths on properties and microstructures of lead-free solder alloys. J Mater Sci: Mater Electron 20, 685–694 (2009). https://doi.org/10.1007/s10854-009-9895-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-009-9895-2