Abstract
The high dielectric constant X8R dielectric materials could be sintered at 1,240 °C by doping 2.5 mol% Pb(Ti,Sn)O3 additives into the BaTiO3 ceramics, with a dielectric constant greater than 3,400 at 25 °C, dielectric loss lower than 2.0% and temperature coefficient of capacitance (TCC) less than ±15% from −55 to 150 °C, which satisfied X8R specification. The effects of Pb(Ti,Sn)O3 on the microstructure and dielectric properties of BaTiO3-based ceramics were investigated. Doped with Pb(Ti,Sn)O3 additives, the partial solid solution was formed between Pb(Ti,Sn)O3 and BaTiO3. Due to the high Curie point of Pb(Ti,Sn)O3, the Curie point of the ceramics was markedly shifted to higher temperature about 150 °C, and the temperature coefficient of capacitance curves was flattened. The increase of the tetragonality (c/a ratio) and the fine microstructure were resulted in the increase of dielectric constant. With Pb(Ti, Sn)O3 content up to 3 mol%, the depression of Ti4+’s polarization and the decrease of the tetragonality (c/a ratio) were resulted in the decrease of dielectric constant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The future developments in high performance multilayer ceramic capacitors, driven by the market requirements, are miniaturization and high volumetric efficiency. Keys to achieve the goal are to increase the capacitance of multilayer ceramic capacitors, which requires the increase in the dielectric constant (ε) of active dielectric layers between internal electrodes [1, 2]. Meanwhile, multilayer ceramic capacitors (MLCCs) have come into use for harsh conditions, such as the engine electronic control unit (ECU) and programmed fuel injection (PGMFI) [1–3]. These modules are subjected to high temperature above 130 °C. MLCCs that only meet the X7R (−55 to +125 °C, temperature coefficient of capacitance TCC ≤± 15% or less) characteristics are not good enough to be used. MLCCs have to satisfy the X8R specification (−55 to +150 °C, TCC ≤± 15% or less) for automotive applications. Therefore, it is most important and critical to research and develop high-ε X8R dielectric materials.
Barium titanate (BaTiO3) was a well-known material for X7R capacitors [4]. The BaTiO3–Nb2O5–Co2O3–Sm2O3 system, capable of sintering in air atmosphere, was known as high-permittivity, temperature-stable materials conforming to X7R specification. The doping mechanism of Nb2O5, Co2O3 in BaTiO3-based system was clear. When Nb2O5 and Co-oxide were used as a pair of dopants, the donor and the acceptor could replace the Ti site by forming the composite cation of [Nb 5+2/3 Co 2+1/3 ]4+, which could produce the core–shell structure [5, 6]. It was very helpful to flatten the ε-T curve. Manganese addition has proved to be effective in reducing dielectric loss (tanδ) in barium titanate compound, and tanδ at room temperature was about 1.0% [7]. Unfortunately, since their Curie point (Tc) was about 130 °C, it was very difficult for BaTiO3–Nb2O5–Co2O3–Sm2O3–MnCO3 system materials to satisfy the X8R characteristics at temperature above 130 °C according to the Curie–Weiss law ε = C/(T−Tc). Therefore, increasing and broadening the Tc was helpful to improve the temperature stability of ε to meet the X8R requirement.
Pb(Ti,Sn)O3(PTS) was an attractive piezoelectric material because of its strong ferroelectricity at room temperature and a high Curie temperature of 296 °C [8, 9]. In addition, PTS can form solid solution with BaTiO3 because of the same pervoskite structure. In our research, it has been found that the Tc of BaTiO3 was greatly increased as high as 150 °C. The high-ε X8R dielectric materials were developed by the addition of PTS to BaTiO3–Nb2O5–Co2O3–Sm2O3–MnCO3 ceramics. The effects of PTS content on the dielectric properties and microstructure were investigated. The aim of this research was to discuss the role of PTS addition and its potential in development of high-ε X8R system for MLCCs applications.
2 Experimental procedures
2.1 Pb(Ti, Sn)O3 preparation
Reagent-grade PbO, TiO2 and SnO2 were used as the starting materials. The solid solution was prepared according to Pb (Ti1−x ,Sn x )O3, x = 0.45. The powders were ball milled in deionized water for 7 h, and dried. The PTS was synthesized at 1,050 °C for 2 h.
2.2 X8R samples preparation
Reagent-grade BaTiO3, Nb2O5, Co2O3, Sm2O3, MnCO3 and synthesized PTS were used as the starting materials. The samples were prepared according to BaTiO3 (calcined at 1,100 °C×2 h) + (2–2.5 mol%) Nb2O5+ (1–2%) Co2O3+ (1–3 mol%) Sm2O3+ (0.5–1.2mol%) MnCO3+ (1.5–3 mol%) PTS. Samples were produced with four different PTS contents, which were 1.5, 2, 2.5 and 3 mol% respectively. The powders were ball milled in deionized water for 8 h, and dried, sieved, then pressed into disks with 15 mm diameter. The disks were sintered at 1,240 °C for 4 h.
2.3 X8R samples measurement
The ε and tanδ were measured by Agilent 4278 Capacitance Meter at 1 KHz, with temperature range of −55 to 160 °C. TCC equaled to(C–C25 °C)/C25 °C, where C and C25 °C represented the capacitance value at measuring temperature and at 25 °C respectively. The surface microstructure of sintered samples was observed with Scanning Electron Microscopy (SEM, Philips XL 30).The core–shell structure was observed with Scanning Electron Microscopy (NanoSEM430, FEI). The phases existed in ceramics were examined using an X-ray diffractometer (XRD, Rigaku 2038X) with Cu Ka radiation (λ = 0.15406 nm) at a step width of 0.02° and a scan rate of 2°/min. The lattice parameters were derived from XRD data by least square fit.
3 Results and discussion
3.1 XRD and SEM analysis
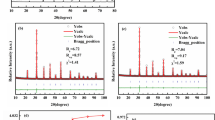
Figure 1 shows X-ray analysis of samples doped with different content of PTS. As shown in Fig. 1, main phase was BaTiO3, and non-ferroelectric phases Ti 0.1Sn0.9O2, Ti9O17, TiO were formed in all samples. Within the XRD resolution limit, the Nb, Co, Sm and Mn elements were not detected in the profile. The phases of specimen with different content of PTS were similar to each other. X-ray analysis indicated that the partial solid solution was formed between BaTiO3 and PTS. However, the PTS phase was not detected in the XRD profile, which meant the solid solubility of PTS in the BaTiO3-based ceramics >3 mol. The diffraction peaks round 45° were separated from each other for four samples, corresponding to the crystal plane of (002) and (200) respectively, which indicated that the crystal structure of four samples was typically tetragonal [2]. The intensity of non-ferroelectric phases was decreased as increasing PTS content.
Figure 2 shows SEM images of samples doped with different content of PTS. Two types of grains were observed in the ceramics. All samples were composed of matrix fine grains and secondary phase grains. According to XRD analysis, the matrix fine grains were BaTiO3, and secondary phase grains were Ti0.1Sn0.9O2, Ti9O17 and TiO non-ferroelectric phases which were formed the liquid phase during the sintering. As shown in Fig. 2c, the sample doped with 2.5 mol% PTS was predominantly composed of fine grains with smooth surface and clear boundaries, although a small amount of strip grains were observed.
For the sample doped with 2.5 mol% PTS, a fine structure in the core and a coarse structure in the shell were observed in Fig. 3, so this sample was the core–shell type.
3.2 The effect of Pb(Ti, Sn)O3 additives on dielectric properties of high dielectric constant X8R BaTiO3-based ceramics
Figure 4a shows temperature dependence of ε for BaTiO3 ceramics doped with different content of PTS sintered at 1,240 °C for 4 h. The Tc of BaTiO3 ceramics was greatly increased to 150 °C by the additive of PTS, while pure BaTiO3 has a Tc of 130 °C. The shift of Tc from 130 to 150 °C was attributed to the replacement of Ba2+ (0.136 nm) with Pb2+(0.118 nm). The melting point of PbO was as low as 886 °C, which meant the Pb–O bonds were weakened in the perovskite ABO3 structure, which induced that the Ti–O bonds were strengthened after PTS were doped to BaTiO3.The interaction between Ti4+ and its near O2− were so strong that Ti4+ could not resume its seat unless the thermal vibration of Ti4+ were enhanced and the ferroelectricity were weakened by high temperature. Hence, Tc was increased by the addition of PTS. On the other hand, in the perovskite ABO3 structure, A-site ion was at the center of oxygen dodecahedron, and there were eight oxygen octahedrons held down directly by A-site ion. In addition, more oxygen octahedrons were pined indirectly by A-site ion. So although a few A-site ions were replaced, vast Ti–O bonds could be influenced. That is to say, Tc was greatly improved although a few A-site ions were replaced by Pb2+ [10]. Meanwhile, the partial solid solution was formed between BaTiO3 and PTS. The Tc of solid solution was determined by the mole percentage of ingredients [11]. The Tc of PTS was as high as 296 °C, so the Tc of BaTiO3 ceramics doped with PTS was shifted to higher temperature.
As shown in Fig. 4a, the similar curve shapes were observed for 1.5–3 mol% PTS doped samples. As the samples show two peaks in their ε-T curves, the core–shell structure was existed in the ceramics. The core–shell structure of the samples doped with 2.5 mol% PTS was observed in Fig. 3. Figure 4b shows the temperature dependence of TCC of the BaTiO3 ceramics with various PTS contents. Doped with 1.5–3 mol% PTS, the TCC of BaTiO3 ceramics was less than ±15% from −55 to150 °C, which satisfied the X8R specification. The ceramics doped with 2.5 mol% PTS exhibited the highest ε (3400) at 25 °C. XRD analysis indicated that non-ferroelectric phases were decreased as increasing PTS content. According to Lichtenecker formula:
So, why the ε was decreased when the content of non-ferroelectric phases was increased was explained well by equation (1).
As shown in Table 1, the lattice parameters and tetragonality (c/a ratio) were derived from XRD data by least square fit. The tetragonality (c/a ratio) was increased as the content of PTS increasing from 1.5 to 2.5 mol%. Because the grain core possessed the tetragonal structure of pure BaTiO3, the tetragonality (the c/a ratio) was associated with the volume fraction of grain core [12]. Doped with 2.5 mol% PTS, the c/a ratio was as high as 1.008173, which suggested that the volume fraction of the tetragonal grain core was increased, whereas the volume fraction of the cubic grain shell was decreased. According to the Lichtenecker formula, the ε of the ceramics was increased accordingly. The fine and homogeneous grains were responsible for good dielectric properties. SEM indicated that fine and homogeneous grains were observed at 25 °C with 2.5 mol% PTS additives, which also gave rise to the increase of ε.
On the other hand, ε was reduced with increasing PTS content up to 3 mol%. Doped with abundant amount of PTS, Pb2+ ion (0.12 nm) substituted for A-site (Ba2+ 0.135 nm) in ABO3 structure. It formed cationic vacancies, for the radius of Pb2+ was much smaller than Ba2+. Those vacancies would result in the shrinkage of crystal lattice in some direction. Meanwhile, Sn4+ (0.069 nm) substituted for B-site (Ti4+ 0.061 nm) in ABO3 structure. Sn4+ possessed larger radius in B-site, so the volume of tin centered eight oxygen octahedrons was increased. Hence, the volume of titanium centered eight oxygen octahedrons was decreased. So the active space for Ti4+ was decreased, and Ti4+ ions were restricted to the center of oxygen octahedrons. Thus Ti4+’s polarization was depressed, then ε was decreased accordingly. As shown in Table 1, the decrease of the tetragonality (c/a ratio) suggested the volume fraction of the tetragonal grain core was decreased, whereas the volume fraction of cubic grain shell was increased. According to the Lichtenecker formula, the ε of the ceramics was decreased accordingly. Moreover, tanδ of four samples was lower than 2.0% at 25 °C, which satisfied X8R specification.
4 Conclusions
The ceramics doped with 2.5 mol% PTS, exhibited the highest ε (about 3,400) at room temperature, which satisfied X8R specification. Doped with PTS additives, the Tc of the ceramics was markedly shifted to higher temperature about 150 °C. The substitution of Pb2+ for the A-sites in BaTiO3 strengthened Ti–O bonds, resulting in the increase of Tc. Because the Tc of the PTS ingredients was as high as 296 °C, the Tc of the ceramics doped with PTS was shifted to higher temperature. XRD analysis proved that as the content of PTS increasing, the non-ferroelectric phases were decreased, and the tetragonality (c/a ratio) was increased, which resulted in the increase of ε. SEM indicated that doped with 2.5 mol% PTS, fine and homogeneous grains were observed, which also gave rise to the increase of ε. With PTS content up to 3 mol%, the substitutions of ions for A-site and B-site, resulted in the shrinkage of crystal lattice in some direction and the decrease in the volume of titanium centered eight oxygen octahedrons. Thus Ti4+’s polarization was depressed, then ε was decreased accordingly. The decrease in the tetragonality (c/a ratio) was also resulted in the decrease of ε.
References
S. Wang, Mater. Lett. 59, 2457 (2005)
B. Li, J. Mater. Sci. 42, 2090 (2007)
M. Du, J. Elec. Mater. 36, 1389 (2007)
Y. Yuan, Mater. Lett. 58, 1959 (2004)
L. Wen, Mater. Lett. 57, 1 (2002)
H. Chazono, J. Am. Ceram. Soc. 83, 101 (2000)
L. Tao, Mater. Lett. 441 (2000)
B.P. Das, Ceram. Trans. 103, 261 (2004)
S. Shirasaki, Bull. Chem. Socie. Japan 47, 568 (2009)
Y. Yuan, J Mater. Sci. Mater. Electron. 20, 157 (2009)
B. Li, Inorganic Dielectric Materials (1986), p. 50
Y. Park, Mater. Lett. 28, 101 (1996)
Acknowledgments
This work was supported by the Tianjin Natural Science Foundation of China under Grant No. 06YFJMJC01000 and the Science Development Foundation of Tianjin University of Technology and Education No. KJ0803.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Su, H., Chen, L. et al. Effect of Pb(Ti, Sn)O3 on the dielectric properties of high dielectric constant X8R BaTiO3-based ceramics. J Mater Sci: Mater Electron 21, 1159–1163 (2010). https://doi.org/10.1007/s10854-009-0038-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-009-0038-6