Abstract
0.9BaTiO3–0.1(Bi0.5Na0.5)TiO3–x mol% NiNb2O6 (x = 0, 0.5, 1.0, 1.5, 2.0, 2.5) ceramics were prepared through traditional solid-state reaction method. The effects of NiNb2O6 doping on the dielectric properties and the microstructure of ceramics were investigated systematically. The results showed that the grain size of the ceramics decreased with the increase of NiNb2O6 doping amount. Ba11(Ni,Ti)28O66+x and Nb2O5 were detected in the X-ray diffractions patterns. The Curie point Tc increased slightly with the increase of NiNb2O6 doping amount. It was interesting to find that the dielectric constant at low temperature of the ceramics first increased and then decreased with the increase of NiNb2O6 concentration. Dielectric temperature stability of 0.9BaTiO3–0.1(Bi0.5Na0.5)TiO3 ceramics greatly depended on NiNb2O6 content. The optimum dielectric properties meeting the X9R specification were obtained in the composition of 0.9BaTiO3–0.1(Bi0.5Na0.5)TiO3 with 1.5 mol% NiNb2O6 sintered at 1200 °C, εr = 1652 and tanδ = 1.8% at room temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recent developments in modern electronic devices have brought new demands for multilayer ceramic capacitors (MLCCs) with higher performances. However, one of the major problems of MLCCs is to raise the upper limit of operating temperature under the premise of keeping high temperature-stable dielectric constant and high reliability [1,2,3]. Although X8R (− 55 to 150 °C, △C/C25°C ≤ ± 15%) MLCCs have been widely studied and reported in many research groups [4,5,6], it is necessary to exploit new ceramics systems which satisfy EIA-X9R specification (− 55 to 200 °C, △C/C25°C ≤ ± 15%) due to the emergence of many new applications working in the environment with broadened high temperature range [7].
The pure BaTiO3 (BT) ceramic has a sharp phase transition peak at Curie temperature (Tc), which seriously deteriorates temperature stability. To improve the temperature stability, there are mainly two methods: (1) Preparing a solid solution with higher Curie temperature by doping an effective Curie temperature shifter in BaTiO3, such as (Bi0.5Na0.5)TiO3 (BNT) [8, 9] or (K0.5Na0.5)NbO3 [10, 11]. For example, the Tc of the solid solution 0.9BT–0.1BNT is above 160 °C [9], much higher than that of pure BT (125 °C); (2) Forming a fine-grained core–shell structure in the BT-based ceramics [12,13,14]. The dielectric temperature characteristics of the ceramics with a core–shell structure could be evaluated using the Lichtenecker formula [15]:
where Vc, Vs and εc, εs are the volume fraction and the relative dielectric constant of the ceramics in core and shell, respectively. At room temperature, the core part is tetragonal ferroelectric phase and the shell part is pseudo-cubic paraelectric phase. By the way, Curie temperature is the temperature at which there will be a phase transition from ferroelectric to paraelectric. The overall dielectric exhibits an insensitive dielectric response to temperature due to the diffuse phase transition caused by the core–shell structure [16]. Therefore the core–shell structure can help to improve the temperature stability of the dielectric properties.
Considering those two methods mentioned above, many researchers pay more attentions to the core–shell structure in 0.9BT–0.1BNT system. Because of the low diffusion velocity in BT, Nb2O5 is the most common dopant used in 0.9BT–0.1BNT system to form the core–shell structure [17,18,19,20]. When Nb2O5 is added, Nb5+ will substitute for Ti4+, acting as a donor, and the substitution process will unexpectedly increase the concentration of defects. The co-doping of donor and acceptor has been taken to solve this problem. But another problem is that most kinds of dopants would decrease Tc. For example, it was reported that the substitution of Ni2+ for Ti4+ caused a decrease of Tc in 0.9BT–0.1BNT–Nb2O5 ceramics [21]. Meanwhile, the addition of Ni2+ can be achieved by the doping of NiNb2O6. Previous research has established that Tc of BT–CaZrO3 ceramics can be promoted slightly with the increase of NiNb2O6 doping amount, and the 1.5 wt%-doped sample showed the optimal dielectric performance meeting the X8R specification [22]. However, the relative study about BT-based ceramics doped with NiNb2O6 is still insufficient.
In this paper, 0.9BT–0.1BNT–x mol% NiNb2O6 ceramics were prepared through solid-state reaction method and the effects of NiNb2O6 on the microstructure and dielectric properties of 0.9BT–0.1BNT ceramics were studied.
2 Experimental procedure
BT–BNT–NiNb2O6 ceramic samples were prepared through traditional solid-state reaction method. NiNb2O6 and BNT powders were pre-synthesized using reagent grade NiO, Nb2O5, Na2CO3, Bi2O3, and TiO2 as raw materials, and calcined at 1000 °C for 2 h and 900 °C for 2 h [19, 23], respectively. BaTiO3 powder with an average size of 300 nm was mixed with the obtained BNT at a molar ratio of 9:1, then calcined at 1050 °C for 8 h to obtain the 0.9BT–0.1BNT solid solution [18]. Subsequently, various amounts of NiNb2O6 (0, 1.0, 1.5, 2.0, 2.5 mol%) were added into the solid solution of 0.9BT–0.1BNT, and then ball-milled with the deionized water for 6 h. After dried, the powders were pressed into discs with 15 mm in diameter and 1 mm in height under the pressure of 4 MPa [22]. These discs were sintered at 1200–1250 °C for 3 h in air with a heating rate of 5 °C/min [18, 24].
The phase structures of the specimens were detected by the X-ray diffraction (XRD) analysis (XRD, Rigaku D/max 2550 PC, Tokyo, Japan, with Cu Kα radiation generated at 40 kV and 40 mA). According to the work of the predecessors [25,26,27,28], XRD patterns of samples were obtained in Bragg–Brentano (θ–2θ) geometry. The microstructures of ceramics were observed by scanning electron microscopy (SEM, MERLIN Compact, Germany). The average grain sizes were analyzed using the NanoMeasurer software. For the dielectric properties measurements, silver electrode paste was coated on the opposing surfaces of the sintered samples and sintered at 850 °C for 30 min for deoxidization. The dielectric properties were measured from − 55 to 200 °C (at a heating rate of 3 °C/min) using LCR meter (HP4278A, HP, USA) at 1 kHz and oscillation level of 1.0 V.
3 Results and discussions
3.1 XRD analysis
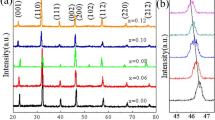
Figure 1 shows the XRD patterns of the ceramic samples with different amount of NiNb2O6. The desired perovskite phase was observed in all samples, and two kinds of secondary phases including Ba11(Ni,Ti)28O66+x and Nb2O5 were detected with the increase of NiNb2O6 doping amount. Ba11(Ni,Ti)28O66+x appeared when the NiNb2O6 doping amount was greater than 2.0 mol%, and the relative concentration increased with the increase of NiNb2O6 addition. It is reported that the valence of Ni in Ba11(Ni,Ti)28O66+x is + 3 [21], which indicates the transformation of some Ni2+ to Ni3+ during the sintering of the ceramics. The appearance of Ni3+ suppress the solubility of Nb5+ in the lattice, therefore we can find the phase of Nb2O5 in the XRD patterns when x = 2.5. For BT, there are two adjacent diffraction peaks around 46°: (002) and (200). As it can be seen from the inset of Fig. 1, the two peaks (002) and (200) are split in the un-doped 0.9BT–0.1BNT ceramic. They gradually merged together by increasing the concentration of NiNb2O6 to 1.5 mol%. However, slightly split was found with the further increase of the concentration. In addition, it is found that the diffraction peaks shift towards lower degree when x was changed from 0 to 1.5. However, the shift cannot be observed when x ≥ 2.0. These results were attributed to the difference in radius of doping ions and substituted ions.
The extrapolated ionic radius of Ni3+ (0.56 Å) is smaller than that of Ti4+ (0.605 Å), and the radii of Ni2+ (0.69 Å) and Nb5+ (0.64 Å) are bigger than that of Ti4+. When the doping amount of NiNb2O6 is less than 2.0 mol%, with the increase of NiNb2O6 substitution, the diffraction peaks shift towards lower degree, which indicated that small amount of NiNb2O6 doping could increase the unit cell volume because of the bigger radii of Ni2+ and Nb5+ than that of Ti4+. The substitution also suppressed the polarization of Ti–O octahedral, as a result, the lattice gradually changed from tetragonal to pseudo-cubic, and (002) and (200) gradually merged together. When the doping amount of NiNb2O6 is greater than 2.0 mol%, since there isn’t any obvious shift of the diffraction peaks with the increase of NiNb2O6 doping amount, the substitution for Ti4+ must include some smaller ions, which should be Ni3+. The substitution of Ni3+ for Ti4+ will lead to a decrease in unit cell volume and strengthening of polarization, which can offset the influence of Ni2+ and Nb5+, thus the shift of diffraction peaks was not obvious, and the lattice gradually changed from pseudo-cubic to tetragonal.
3.2 SEM analysis
Surface morphologies of the 0.9BT–0.1BNT–x mol% NiNb2O6 samples are shown in Fig. 2. From Fig. 2 we can see that all the samples are highly densified. Moreover, the coexistence of smaller grains and larger grains is confirmed, and the number of smaller grains increased with the doping amount of NiNb2O6. Figure 3 shows the effect of NiNb2O6 content on the average grain size of ceramics, from which we can know that the average grain sizes of ceramics doped with NiNb2O6 are much smaller than that of un-doped BT–BNT ceramic. However, there are some flake-like grains formed when x ≥ 2.0. Here we just show the flake-like grains in 0.9BT–0.1BNT–2.5 mol% NiNb2O6 sample in Fig. 4a to observe the appearance of them. As shown in Fig. 4b, the EDS result of the flake-like grain indicated that it is composed of four elements, Ba, Ti, Ni and O, which corresponds to the Ba11(Ni,Ti)28O66+x phase in XRD patterns.
3.3 Dielectric properties
The temperature dependences of dielectric properties of 0.9BT–0.1BNT–x mol% NiNb2O6 ceramics (x = 0.5, 1.0, 1.5, 2.0, 2.5) are displayed in Fig. 5, from which we can see that with the increase of NiNb2O6 content, two dielectric constant peaks appeared, as marked by the two arrows. The high temperature peak and the low temperature peak are corresponding to the core phase and the shell phase, respectively, indicating the existence of core–shell structure [29]. However, the low temperature peak was hard to be observed when the doping level was 0.5 mol%, 2 mol% and 2.5 mol%. When doped level was 0.5 mol%, the NiNb2O6 doping amount is too low so that the volume fraction of shell region is very small. Therefore the low temperature peak is hard to be observed. With the increase of NiNb2O6 content, the two peaks were broadened due to the diffuse phase transition behaviors, and the peak of shell region is gradually obscured by the low temperature tail of the Curie peak. So it is hard to be observed either when x is greater than 1.5. In addition, the dielectric constant peak of core phase Tc shifted towards the higher temperature with the increase of doping amount of NiNb2O6, just as shown as dash line in Fig. 5. Given that the ceramics doped with 0 and 0.5 mol% NiNb2O6 do not meet the requirements of X9R specification, ΔC/C25°C of these two samples are not shown in Fig. 5b for clarity. Table 1 gives the dielectric properties of 0.9BT–0.1BNT–x mol% NiNb2O6 ceramics, from which it can be known that all the dielectric loss detected are below 5%, and the temperature dependence of the samples changed with NiNb2O6 doping amount accordingly. It should be noted that 0.9BT–0.1BNT–1.5 mol% NiNb2O6 sample presents the most flattened temperature dependency, satisfying the X9R specification, and the dielectric properties are εr = 1652, tanδ = 1.8%.
From Fig. 5 we can see that for the 0.9BT–0.1BNT–x mol% NiNb2O6 samples, the εr–T curves changes in a clockwise direction with the increase of NiNb2O6 doping amount up to 1.5 mol%. In other words, dielectric constant decreases at high temperature end, and increases at low temperature end. This is due to the decrease of volume ratio of core and the increase of volume ratio of shell in the grain. However, the curves show an overall decline with the further increase of NiNb2O6 amount, which is related to the grain size and second phase. It is apparent from Fig. 3 that the average grain size decreased with the increase of NiNb2O6 content, which is caused by pinning effects [30]. The shell “NiNb2O6-doped BT–BNT” acted as diffusion barrier and prevented BT–BNT core from grain boundary diffusion/migration and grain growth. The decreased grain size caused the increase of the grain boundary area per unit volume. Actually, the width of grain boundary is not negligible, and it could be considered fixed [31]. Therefore the volume ratio of the grain boundary in the ceramics increased with the increase of NiNb2O6 content. That is to say, a great deal of non-ferroelectric phase is introduced in ferroelectric ceramics with the increase of NiNb2O6 content, which reduces the dielectric constant of the entire ceramic system accordingly. The formation of second phase also shows impact on the dielectric properties. Since Ba11(Ni,Ti)28O66+x has similar synthesis condition as Ba11(Fe,Ti)28O66+x, which has a rather low dielectric constant (< 100) and dielectric loss [32], it is reasonable Ba11(Ni,Ti)28O66+x has a low dielectric constant too. The combined effects of grain size and Ba11(Ni,Ti)28O66+x result in the decrease of dielectric constant when x is greater than or equal to 2.0.
Compared with the pure 0.9BT–0.1BNT, the Tc of the 0.9BT–0.1BNT–x mol% NiNb2O6 ceramics decreased. However, the Tc show an increasing behavior with the increase of NiNb2O6 content. There are three influence factors on Tc [33]: (1) the average ionic radium, here we just discuss RB-site; (2) the statistical variance in the distribution of the radii σ2, which can be used to describe the local strain field. RB-site and σ2 can reflect the influence of lattice mismatch; (3) electrovalence mismatch between the doped ions and the matrix ions, which can reflect the local electric field. However, this mechanism is just applicable when the ion could be considered spherical [34]. The electrovalence mismatch and larger RB-site will lead to a drop of Tc, while larger σ2 shows opposite effect. Electrovalence mismatch such as Ni2+ substituting for Ti4+ may introduce oxygen vacancies, which will increase the phase stability of cubic phase [35]. For BT-based ceramics, with the increase of concentration of doping, the RB-site, σ2, and electrovalence mismatch have been changed, which leads to the change of local strain field and local electric field. Therefore the diffuse phase transition behavior was affected, resulting in the change of the Tc.
When x ≤ 1.5, Ni2+ and Nb5+ cations diffuse into the crystal lattice in the form of (Ni1/32+Nb2/35+)4+ with an effective radius of 0.66 Å which is larger than that of Ti4+, and a valence of + 4 which is identical to Ti4+. In this condition, electrovalence mismatch does not exist, and RB-site and σ2 both increase with the doping amount of NiNb2O6. With the supporting of the theory mentioned above, the change of Tc reveals that the absence of electrovalence mismatch and the larger σ2 show more impact than RB-site and result in the increase of Tc. When x is greater than 2.0, the presence of Ni3+ is confirmed according to the former analysis, and the shift of the diffraction peaks is little. If Ni3+ and Nb5+ still diffuse into the crystal lattice according to the molar ratio of 1:2, the diffraction peaks should have shifted towards lower degree because the effective radius of (Ni1/33+Nb2/35+)13/3+ (0.613 Å) is larger than that of Ti4+, which is not consistent with the fact. A possible explanation for this might be that Ni3+ and part of Nb5+ diffuse into the crystal lattice in the form of (Ni1/23+Nb1/25+)4+. The rest of Nb5+ appears in the form of Nb2O5, which has been proved by XRD in Fig. 1. (Ni1/23+Nb1/25+)4+ has an effective radius of 0.60 Å, just a little smaller than that of Ti4+, and an identical valence to Ti4+, so that the electrovalence mismatch is absent. RB-site decreased with the increases of (Ni1/23+Nb1/25+)4+ content, while σ2 has little change. Therefore the substitution of (Ni1/23+Nb1/25+)4+ for Ti4+ made the Tc have a potential of further increase due to the absence of electrovalence mismatch and the smaller RB-site. The collective effect of (Ni1/32+Nb2/35+)4+ and (Ni1/23+Nb1/25+)4+ on the ceramics results in the increase of Tc.
The Nb5+ as well as Ni2+ substituting for Ti4+ in 0.9BT–0.1BNT system leads to the monotonous reduction of Tc, but the doping of NiNb2O6 can promote Tc. This may suggest that we can use ion combinations with the same valence and similar radius to Ti4+ to improve the Tc of BT-based ceramics.
4 Conclusion
The effects of NiNb2O6 content on microstructures and dielectric properties of 0.9BT–0.1BNT ceramics were studied in this paper. The results show that the doping of NiNb2O6 can suppress the grain growth. In spite of the facts that Tc of the doped samples are lower than that of un-doped 0.9BT–0.1BNT ceramic, Tc shows an increasing behavior with the increase of NiNb2O6 content. Dielectric constant at low temperature first increased and then decreased. The change of dielectric constant can be related to the cooperating influence of core–shell structure and grain size. And the increase of Tc can be explained by the change of RB-site, σ2 and electrovalence mismatch, which suggested a possible method to use proper ionic combinations to increase Tc. 1.5 mol% NiNb2O6-doped specimen met X9R specifications and exhibited excellent dielectric properties with a dielectric constant of 1652 and a dielectric loss of 1.8% at room temperature.
References
C. Groh, K. Kobayashi, H. Shimizu, Y. Doshida, Y. Mizuno, E.A. Patterson, J. Rödel, J. Am. Ceram. Soc. 99(6), 2040–2046 (2016)
S. Wang, H. He, H. Su, J. Mater. Sci.: Mater. Electron. 23(10), 1875–1880 (2012)
Z. Liu, H. Fan, S. Lei, X. Ren, C. Long, J. Eur. Ceram. Soc. 37(1), 115–122 (2017)
S. Wang, H. He, H. Su, J. Mater. Sci.: Mater. Electron. 24(7), 2385–2389 (2013)
L. Li, Y. Liu, M. Wang, N. Zhang, J. Chen, J. Mater. Sci.: Mater. Electron. 25(10), 4252–4258 (2014)
X. Huang, H. Liu, H. Hao, Z. Wang, W. Hu, Q. Xu, J. Mater. Sci.: Mater. Electron. 26(5), 3215–3222 (2015)
X. Huang, W. Zhang, J. Xie, Q. Xu, L. Zhang, J. Mater. Sci.: Mater. Electron. 28(5), 4204–4210 (2017)
Z. Shen, X. Wang, L. Li, J. Mater. Sci.: Mater. Electron. 28(4), 3768–3773 (2017)
G. Yao, X. Wang, Y. Wu, L. Li, J. Am. Ceram. Soc. 95(2), 614–618 (2012)
Z. Hu, B. Cui, M. Li, L. Li, J. Mater. Sci.: Mater. Electron. 24(10), 3850–3855 (2013)
Z. Hu, B. Cui, S. Jing, Y. Wang, Mater. Lett. 113, 167–169 (2013)
T. Wang, H. Hao, M. Liu, D. Zhou, Z. Yao, M. Cao, H. Liu, J. Am. Ceram. Soc. 98(3), 690–693 (2015)
M. Liu, H. Hao, W. Chen, D. Zhou, M. Appiah, B. Liu, Z. Zhang, Ceram. Int. 42(1), 379–387 (2016)
R. Ma, B. Cui, M. Shangguan, S. Wang, Y. Wang, Z. Chang, Y. Wang, J. Alloys Compd. 690, 438–445 (2017)
D. Hennings, G. Rosenstein, J. Am. Ceram. Soc. 67(4), 249–254 (1984)
R. Farhi, M. El Marssi, A. Simon, J. Ravez, Eur. Phys. J. B 18(4), 605–610 (2000)
Z.B. Shen, X.H. Wang, D.S. Song, L.T. Li, Adv. Appl. Ceram. 115(7), 435–442 (2016)
G. Yao, X. Wang, Y. Zhang, Z. Shen, L. Li, J. Am. Ceram. Soc. 95(11), 3525–3531 (2012)
Y. Zhang, S. Gao, B. Zhang, J. Mater. Sci.: Mater. Electron. 26(5), 2709–2712 (2015)
Y. Sun, H. Liu, H. Hao, L. Zhang, S. Zhang, Ceram. Int. 41(1), 931–939 (2015)
Y. Sun, H. Liu, H. Hao, Z. Song, S. Zhang, J. Am. Ceram. Soc. 98(5), 1574–1579 (2015)
L. Li, J. Yu, N. Zhang, J. Ye, J. Mater. Sci.: Mater. Electron. 26(12), 9522–9528 (2015)
C.Y. You, Y.C. Zhang, J. Mater. Sci.: Mater. Electron. 27(6), 6606–6613 (2016)
S.F. Wang, Y.F. Hsu, Y.W. Hung, Y.X. Liu, Appl. Sci. 5(4), 1221–1234 (2015)
R. Ma, Y. Wang, B. Cui, Y. Wang, J. Mater. Sci.: Mater. Electron. 28(15), 10986–10991 (2017)
X. Chen, X. Li, G. Huang, G. Liu, X. Yan, J. Mater. Sci.: Mater. Electron. 28(22), 17278–17282 (2017)
Y. Wang, B. Cui, M. Shangguan, S. Wang, J. Mater. Sci.: Mater. Electron. 29(8), 6369–6376 (2018)
R. Guinebretière, X-Ray Diffraction by Polycrystalline Materials, 1st edn. (Wiley, London, 2007), pp. 94–97
Y. Liu, B. Cui, Y. Wang, J. Am. Ceram. Soc. 99(5), 1664–1670 (2016)
R. Berthelot, B. Basly, S. Buffière, J. Majimel, G. Chevallier, A. Weibel, J. Mater. Chem. C 2(4), 683–690 (2014)
M.H. Frey, Z. Xu, P. Han, D.A. Payne, Ferroelectrics 206(1), 337–353 (1998)
T.A. Vanderah, T. Siegrist, R.S. Roth, A.P. Ramirez, R.G. Geyer, Eur. J. Inorg. Chem. 2004(12), 2434–2441 (2004)
S. Liu, L. Zhang, J. Wang, Y. Zhao, X. Wang, Ceram. Int. 43(14), 10683–10690 (2017)
A. Berenov, F. Le Goupil, N. Alford, Sci. Rep. 6, 28055 (2016)
Y. Sun, H. Liu, H. Hao, S. Zhang, J. Am. Ceram. Soc. 99(9), 3067–3073 (2016)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 61671323).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiao, M., Sun, H., Wei, Y. et al. Microstructure and dielectric properties of BaTiO3–(Bi0.5Na0.5)TiO3–NiNb2O6 ceramics. J Mater Sci: Mater Electron 29, 17689–17694 (2018). https://doi.org/10.1007/s10854-018-9874-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9874-6