Abstract
Electroless copper plating of polyester fabrics was demonstrated in the present investigation. The electroless Cu plating process on polyester fabric was modified by replacing the conventional PdCl2 activator with an AgNO3 activator to reduce the overall cost of the plating process. Both uncoated and Cu-coated polyester fabrics were characterized by the scanning electron microscope (SEM), energy dispersive spectroscopy (EDX), X-ray diffraction analysis (XRD) and X-ray photoelectron spectroscope (XPS). Relatively uniform and continuous plating was obtained under the given plating conditions. The possible mechanism of electroless copper plating of polyester fabrics utilizing an AgNO3 activator was suggested. The electromagnetic interference (EMI) shielding effectiveness (SE) was also evaluated to study the shielding behavior of copper-plated polyester fabrics. The results demonstrated that copper-plated polyester fabrics can be applied for EMI shielding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, interest has gradually increased in the deposition of metallic layers on polymers for either decorative or functional purposes in applications such as food packaging, microelectronics packaging and coatings for electromagnetic interference (EMI) shielding and wear protection [1–3]. Thus, the deposition of a metallic coating on these polymers enhances their range of application and creates a considerable added value. Conductive textiles, which are coated with aluminum, copper, silver and nickel, are important types of material for preventing electromagnetic interferences. Currently, developed metal coating techniques are metal foil and laminates, conductive paints and lacquers, sputter coating, vacuum deposition, flame and arc spraying, and electroless plating [4–11].
Among them, electroless plating is probably a preferred way to produce metal-coated textiles. The electroless deposition method uses a catalytic redox reaction between metal ions and dissolved reduction agent. With its remarkable advantages, such as low cost, easy formation of a continuous and uniform coating on the surface of substrate with complex shapes, it can be performed at any step of the textile production, such as yarn, stock, fabric or clothing [12].
With regards to the high conductivity of copper, electroless copper plating is currently used to manufacture conductive fabrics. Electroless copper plating on fabrics has been studied by some researchers [13–15]. However, in these investigations, the electroless copper plating is obtained through the usage of conventional sensitization and activation steps involving costlier activators viz. palladium chloride (PdCl2). As a result, the overall cost of the electroless process for plating Cu on polyester fabric surfaces is still a major problem in commercialization. There lacks open literature with respect to electroless metal plating of polyester fabrics using cost-effective chemicals. In this study, the costlier PdCl2 activator was replaced by the less expensive AgNO3 activator without affecting the properties of copper plating. Surface characteristics of copper-plated polyester fibers were investigated in detail through the measurements of the scanning electron microscopy (SEM), Energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). EMI shielding effectiveness of copper-plated fabrics was also evaluated.
2 Experimental
2.1 Materials
Plain weave 100% polyester fabrics (47 × 40 counts/cm2, 84 g/m2) were used as the substrate. The size of each specimen was 10 cm × 10 cm.
2.2 Chemicals
The chemicals used for the electroless copper plating included SnCl2 (anhydrous min. 99.9%), HCl (37%), AgNO3(99.9%), NaOH (99.3%), NH4OH (28%), HCHO (37%) CuSO4 · 5H2O (99%) and NaKC4H4O6 · 4H2O (99.9%). All reagents were of analytical grade.
2.3 Procedure
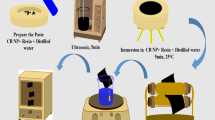
Electroless copper plating was carried out through multi-step processes, including pre-treatment, sensitization, activation, electroless copper plating, post-treatment for stopping copper reduction, rinsing and drying.
All fabric samples were subsequently rinsed with 5% detergent at room temperature for 20 min. The samples were then rinsed in deionized water. Surface sensitization was conducted by immersing the samples into an aqueous solution containing 10 g/L SnCl2 and 40 mL/L 38% HCl acid at 25 °C for 10 min. The specimens were subsequently rinsed in deionized water and activated by immersing them into a solution containing AgNO3 (10 g/L) and 28% NH4OH (10 mL/L) at 25 °C for 20 min. Afterwards, the specimens were rinsed in a large volume of deionized water for more than 5 min to prevent contamination of the plating bath. The specimens were consequently immersed in the electroless copper plating bath at 30 °C for 20 min. The bath was composed of 15 g/L CuSO4 · 5H2O, 20 mL/L HCHO (37% aqueous solution), 40 g/L NaC4H4O6 · 4H2O, 10 g/L NaOH. In the post-treatment stage, the samples were rinsed with deionized water at 40 °C for 20 min and dried in an oven at 60 °C. All the copper-plated fabrics were conditioned in accordance to the ASTM D1776-04 before measurement.
2.4 Characterization
A Field Emission Scanning Electron Microscope (FESEM, JSM-6335F) was used to characterize the surface morphology of the deposits. The chemical composition of the copper deposits was determined using an energy dispersive X-ray (EDX) analysis that was attached to the SEM. The crystal structure of the copper-plated fabric was investigated using X-ray diffraction (XRD, Cu Kα radiation and graphite filter at 40 kV and 40 mA). A X-ray photoelectron spectroscopy analysis was performed to characterize the surface chemical structure of the copper-plated polyester fabrics by using the Shengyang SKL-12 electron spectrometer equipped with a VG CLAM 4 MCD electron energy analyser. MgK radiation (1253.6 eV) was obtained with an accelerating voltage of 12 kV and an emission current of 20 mA.
EMI shielding effectiveness (SE) was obtained by following ASTM D 4935-99 using a vector network analyzer (Agilent-E8363A) that was equipped with a synthesized frequency source and a scattering parameter (S-parameter) test set over a frequency range of 2–18 GHz.
3 Results and discussion
3.1 SEM/EDX analysis
The surface morphology of thin metallic films may affect their electrical, mechanical, and optical properties. SEM micrographs with different magnifications of uncoated and copper-plated polyester fibers are presented in Fig. 1. In comparison with Fig. 1a, it was obvious that after copper plating, the surface of polyester fibers had significantly changed as shown in Fig. 1b. Polyester fibers were uniformly covered with dense copper particles which were clearly visible and the copper particles were well dispersed on fiber surfaces with the present electroless plating method. The uniform coating was also evident at high magnification (Fig. 1c). The enlarged image of the product also indicates that the copper plating is composed of needle-shaped copper particles. The formation of needle-shaped particles may be explained in the following equation. As well known, the electroless copper plating reaction may be represented by:
Equation 1 indicates that copper ions are reduced to metallic copper with high purity by formaldehyde in the alkaline solution, at the same time as hydrogen gas release. The nano-sized spherical copper particles, which are originally produced at the interface of samples, possess high surface energy and have a strong tendency to aggregate during copper particle deposition. With the influence of the rapid hydrogen release at the interface, some neighboring copper particles tend to aggregate along the gas flow and grow in lines. Thereby, needle-shaped particles are formed [16].
The EDX analysis of uncoated and copper-plated polyester fibrics are presented in Fig. 2a and b. The results showed the prominent presence of Cu within the coating. This indicates a successful copper plating of polyester fabrics through a modified electroless plating process that utilizes the AgNO3 activator. The strong oxygen peak, which was observed for polyester fabrics, was absent in Fig. 2b, suggesting minimum copper oxide formation only within the bulk of the coating and the deposition of pure metallic Cu0. No impurities are observed in the copper plating, within the resolution limit of EDX, which further indicated a high purity copper plating that was obtained using the modified electroless plating technique. Moreover, the substrate elements are also not observed in the EDX spectrum (Fig. 2b), suggesting deposition of a relatively thick copper plating.
The SEM and EDX results indicated that electroless copper plating using an AgNO3 activator was effective in modifying the micro-structure of the polyester fabrics through successful copper depositing.
3.2 XRD analysis
Figure 3 shows the X-ray diffraction patterns of copper-plated polyester samples. The four major strong characteristic peaks of the copper-plated polyester sample at 2θ = 43.4°, 50.3°, 74.2° and 90.1° corresponded to the crystal faces of (111), (200), (220) and (311) of copper, respectively. The copper oxide phase was not detected in the deposits.
Based on the XRD results, the crystal size of the coatings was determined from the broadening of the diffraction peak by employing the Scherrer formula as expressed by Eq. 2:
where t is the crystal size, λ is the X-ray wavelength corresponding to Cu Kα radiation (0.154056 nm), θ is the diffraction angle, B is the full width half maximum (FWHM) of the diffraction peak at 2θ, and n is the Scherrer constant as 0.89 [17]. According to the Scherrer equation, the average size of copper particles was 37.13 nm with respect to the Cu (111) main peak.
The XRD patterns identified by the PDF card of the JADE-SCAN software revealed that the deposited copper film exhibited a characteristic face-centered cubic crystalline structure, implying that the copper-plated polyester fabrics had a perfect conductivity property.
3.3 XPS analysis
The XPS spectra of the copper-plated polyester sample could provide further information about the structure and chemical state of copper films and copper composite films as shown in Fig. 4. Figure 4a shows a typical XPS wide scan spectrum, indicating that all the standard photoelectron lines of elemental Cu were present: Cu 3s, Cu 3p, Cu 2p, O 1s, and C 1s. In the spectrum, only the Cu element with a small amount of O and C elements was detected on the fabric surface, which indicated that a thick coating developed on the fabric surface.
The carbon signal from the surface came from the contaminants and the oxygen mainly from the air. The copper-plated polyester fibers were extremely reactive towards atmospheric oxygen. This affinity of the Cu coating towards oxygen is attributed to the large specific surface area of fine coating.
The peak, located at around 335 eV, is characterized as the Cu LMM Auger line. A narrow XPS scan of Cu 2p within the binding energy (BE) interval of 925–970 ev is shown in Fig. 4b. The Cu 2p spectrum predicted a doublet separation of the Cu 2p spectral line and characterization by a binding energy of 932.2 (2p3/2) and 952.4 eV (2p1/2) with satellite shake-up peaks. The Cu 2p3/2 and 2p1/2 intensities of shake-up satellites approach that of the main line with satellite intensity at around 944.0 and 963.8 eV, while the doublet separation of the Cu 2p spectral line had the separation energy of 20.2 eV. The spectral lines of the Cu 2p3/2 and 2p1/2 were close to the values reported in the literature [18]. Figure 4c shows the spectral line of O 1s at 531.55 eV, which is due to the atmospheric oxygen at the Cu surface. The analyses of Cu-coated fibers showed that there was a relatively uniform metallic Cu layer on the fiber surface, which is extremely reactive towards atmospheric oxygen.
3.4 Mechanism of electroless copper plating of polyester fabrics
The mechanism of electroless Cu-plating of polyester fabrics that utilize the AgNO3 activator is described in the following passages.
It is observed that after putting the polyester fabrics in an acidic SnCl2 bath, Sn2+ ions are adsorbed onto the particle surface, forming a uniform layer [19]. These sensitized polyester fabrics are then inserted into the activation bath, which is prepared by dissolving AgNO3 in aqueous ammoniac solution. This results in the formation of [Ag (NH3)2]+ ions in the activation bath [20, 21], which becomes adsorbed on the fabric surface following the addition of sensitized fabrics to the activation bath. However, Sn2+ ions that are present on the sensitized fabric surface immediately reduces [Ag(NH3)2]+ to Ag0 according to the following reaction:
The deposited Ag0 then acts as a catalyst for the subsequent Cu deposition in the electroless plating bath. It is known that the electroless copper plating process is electrochemical in nature [22, 23]. The beginning of the copper deposition is controlled by the anodic processes. Under the catalytic action of metallic Ag0 clusters adsorbed on the fabric surface, Cu2+ ions are deposited onto the catalytic silver surface by capturing electrons that are furnished by the reducing agent (HCHO) via the following chemical reactions:
Anodic reaction:
Cathodic reaction:
In the reaction initiation, the metallic Ag0 clusters act as nucleation sites for copper deposition. In aqueous alkaline solutions (usually pH > 11), formaldehyde (HCHO) adsorbed on the catalytic Ag0 surfaces is easily oxidized to yield HCOO−, the activated hydrogen atom (•H) and release electrons (Anodic reaction), whereas Cu ions in the plating bath are reduced to metallic Cu0 by the electrons generated through the oxidation of HCHO (cathodic reaction). The combination of two activated hydrogen atoms will be responsible for part of the observed gas evolution. Once the Cu deposition was initiated, the deposited Cu atoms themselves acted as self-catalysts for further Cu deposition and a well-developed copper plating on the polyester fabric surface was then obtained via this electroless plating process.
3.5 Shielding effectiveness
The EMI shielding effectiveness value expressed in dB was calculated from the ratio of the incident to transmitted power of the electromagnetic wave in the following Eq. 5:
where P 1 (E 1) and P 2 (E 2) are the incident power (incident electric field) and the transmitted power (transmitted electric field), respectively [24].
Figure 5 indicates the shielding effectiveness (SE) of the uncoated and coated polyester fabric. As a result, the SE of polyester fabrics was almost zero at the frequencies 2–18 GHz. However, SE of copper-coated polyester fabric was above 40 dB and the tendency of SE kept similarity at the frequencies 2–18 GHz. The results indicated that the electroless Cu coated polyester fabric effectively attenuated penetrability of electromagnetic waves using the AgNO3 activator. The copper-coated polyester fabric has a practical usage for many EMI shielding application requirements.
4 Conclusion
Electroless Cu deposition on polyester fabrics using the AgNO3 activator has been demonstrated in this paper. The deposited copper films were subjected to a detailed analysis by SEM, EDX, XPS and XRD. The results showed that there was a relatively dense, uniformly distributed metallic Cu layer on the fabric surface. The film is composed of needle-shaped copper particles and characteristics of a face-centered cubic crystalline structure. The average crystal size of the coatings was found to be 37.13 nm. The AgNO3 activator is a cost-effective replacement for the conventional PdCl2 activator in the electroless copper plating of polyester fabrics. The study demonstrated that copper-plated polyester fabrics are considered as effective EMI shielding materials. AgNO3 could be a potential alternative to the relatively expensive PdCl2 catalyst for electroless copper plating.
References
G.O. Mallolry, J.B. Hajdu, Electroless Plating: Fundamentals and Applications (American Electroplaters and Surface Finishers Society, Orlando, 1990)
K.L. Mittal, Metallized Plastics; 5 and 6 Fundamental and Applied Aspects (VSP, Utrecht, 1998)
L.T. Romankiw, Electrochim. Acta 42, 2985 (1997)
Innovation 128, Technology Trend, Electromagnetic Interference shielding-A Materials Perspective (Innovation 128, S. A. Paris, 1996)
M. Farsi, Interference Technol. Engr. Master, 228 (1988)
O.S. Kwon, J.C. Jung, Y.H. Yoo, Polymer (Korea) 7(6), 342 (1983)
S. Yasufuku, IEEE Electr. Insul. M. 6(6), 947 (1995)
W.C. Smith, J. Coat. Fabrics 17, 243 (1988)
K. Bula, J. Koprowska, J. Janukiewicz, Fibers Text. East. Eur. 14(5), 75 (2006)
S.Q. Jiang, E. Newton, C.W.M. Yuen, C.W. Kan. Text. Res. J. 76, 57 (2006)
C.W.M. Yuen, S.Q. Jiang, C.W. Kan, W.S. Tung. Appl. Surf. Sci. 253, 5250 (2007)
E.G. Han, K.W. Oh, E.A. Kim, J. Korean Soc. Cloth. Text. 23(5), 694 (1995)
E.G. Han, E.A. Kim, K.W. Oh, Synth. Met. 123, 469 (2001)
X.P. Gan, Y.T. Wu, L. Liu, B. Shen, W.B. Hu. Surf. Coat. Technol. 201, 7018 (2007)
E.A. Kim, E.G. Han, K.W. Oh, J.G. Na, J. Appl. Phys. 87(9), 4984 (2000)
L.N. Xu, K.C. Zhou, H.F. Xu, Appl. Surf. Sci. 183, 58 (2001)
B.E. Warren, X-ray Diffraction (Dover, New York, 1990)
Y.S. Kim, J.H. Shin, J.H. Cho, Surf. Coat. Technol. 200, 5760 (2006)
S. Shukla, S. Seal, J. Akesson, R. Oder, R. Carter, Z. Rahman, Appl. Surf. Sci. 80, 35 (2001)
S. Shukla, S. Seal, D. Zhou, S. Schwarz, J. Nanosci. Nanotech. 1, 407 (2001)
S. Shibata, K. Aoki, T. Yano, M. Yamane, J. Sol-Gel Sci. Technol. 11, 279 (1998)
S. Shukla, S. Seal, Z. Rahaman et al., Mater. Lett. 57, 151 (2002)
H.B. Dai, H.X. Li, F.H. Wang, Surf. Coat. Technol. 201, 2859 (2006)
C.R. Paul (ed.), Introduction of Electromagnetic Compatibility (Wiley, New York, 1992)
Acknowledgements
The authors acknowledge the financial support of The Hong Kong Polytechnic University. Part of the experimental work was carried out in the Materials Research Center of The Hong Kong Polytechnic University, to which the authors would like to express their gratefulness.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, R.H., Jiang, S.Q., Yuen, C.W.M. et al. An alternative process for electroless copper plating on polyester fabric. J Mater Sci: Mater Electron 20, 33–38 (2009). https://doi.org/10.1007/s10854-008-9594-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-008-9594-4