Abstract
Polyester fabric was catalyzed with Nickel(II) acetylacetonate (Ni(acac)2) in supercritical carbon dioxide (scCO2) prior to electroless copper plating . The nickel activated polyester fibers were then characterized by scanning electron microscopy and X-ray photoelectron spectroscopy. Deposited weight, surface morphology, crystal structure, surface resistance, electromagnetic interference shielding effectiveness and thermal loss of the copper plated polyester fabrics catalyzed with nickel in scCO2 were investigated. The results indicate that the amount of copper deposited onto polyester fabric catalyzed with Ni(acac)2 in scCO2 is much more than that without scCO2. Deposition rate of copper particles onto polyester fabric increases with increased temperatures of scCO2. The copper particles are uniformly coated on the polyester fibers. The surface resistance of the copper plated fabric is 50 mΩ/sq when the temperature of scCO2 is increased to 90 °C. The electromagnetic interference shielding effectiveness of the copper plated polyester fabric is 60–80 dB at frequencies ranging from 2 to 18 GHz. These results suggest that catalysis in scCO2 for electroless copper plating provides polyester fabric with excellent electrical conductivity, surface resistance and electromagnetic interference shielding effectiveness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, interest has gradually increased towards the deposition of metals on textiles for either decorative or functional purposes in applications such as food packaging, microelectronics packaging and coatings for electromagnetic interference (EMI) and protective wear [1,2,3]. Thus, the deposition of metals on textiles enhances the range of application and creates considerable added values [4,5,6]. Electroless plating is the preferred method to produce metal coated textiles due to its advantages such as low cost and ease of formation of coating onto the surface of a substrate with complex shapes [7,8,9,10,11]. Electroless copper plating has been frequently used to coat metals on textiles because it provides excellent electrical conductivity, is low in cost and allows deposit uniformity [12, 13]. However, a catalytic reaction is necessary to carry out in electroless copper deposition, which is carried out in an aqueous solution.
Therefore, there has been immense intense in the use of supercritical carbon dioxide (scCO2) in the last few decades because not only is it an environmentally friendly solvent (recyclable), but it is also low in cost, nontoxic, inert, and noninflammable [14]. Even more interestingly, it is possible to modify textiles by using scCO2 because of the unique properties, such as low viscosity, high diffusivity, solubility and zero surface tension [15]. Recently, scCO2 has been used as both a solvent to infuse metal complexes into polymers and a plasticizer to soften the surface of polymers and to increase the adhesion between the metal layers and the polymeric substrates [16, 17]. ScCO2 is extensively used to dye synthetic fibers [18] and inject UV stabilizers into PP fabrics [19]. Currently, palladium (Pd) complexes, such as palladium bis-hexafluoroacetylacetonate (Pd(hfa)2) and palladium bis-acetylacetonate (Pd(acac)2), are used as catalysts, and commonly infused into various kinds of fibers including cotton and polypropylene fibers in scCO2 for electroless deposition [20, 21]. However, there are no reports on the impregnation of a palladium-free organometallic complex into fabric with scCO2 prior to electroless plating.
In this study, an alternative activation method with the impregnation of an organometallic complex, nickel(II) acetylacetonate (Ni(acac)2), into polyester fabric with scCO2 to initiate electroless copper plating on polyester fabric has been developed. The deposition weight, surface morphology and crystal structure of the copper plated polyester fabric were investigated. Then the effect of the temperature of scCO2 on the deposition weight and surface resistance of the copper plated polyester fabrics was examined. Following that, the EMI SE of the copper plated polyester fabric was evaluated.
The composition and chemical structure of the Ni(acac)2 activated polyester fabric was also examined by using X-ray photoelectron spectroscopy (XPS). The surface morphology, chemical composition, and crystal structure of the copper plated polyester fabrics were characterized by scanning electron microscopy (SEM) and X-ray diffraction (XRD). Surface resistance, EMI SE and adhesive strength of the copper plated polyester fabric were evaluated.
2 Experimental
2.1 Materials
Plain weave 100% polyester fabric (47 × 40 counts/cm2, 84 g/m2) in white color was used as the substrate. The polyester fabric was ultrasonically washed in a mixture of acetone and alcohol for 30 min and dried in air for use. The carbon dioxide gas had a purity greater than 99.9%. Ni(acac)2 was purchased from Xiya Reagent Co., Ltd. All reagents were of analytical grade and used without further purification.
2.2 Electroless copper plating
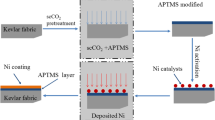
A schematic of the electroless copper plating on the polyester fabric activated with Ni(acac)2 via scCO2 process is shown in Scheme 1.
The activation process was performed in a scCO2 apparatus. Ni(acac)2 (0.1 g) was dissolved by a small amount of alcohol and then transferred into a solvent vessel. The cleaned fabrics were rolled and introduced into a reaction vessel. After the desired temperature (90 °C) was reached, liquid CO2 was added until the desired pressure (10 MPa) was reached. The activation of the polyester fabric was carried out with Ni(acac)2 in scCO2 medium at 10 MPa for 20 min. Finally, the system was slowly depressurized and allowed to cool to room temperature. The polyester fabrics activated with (Ni(acac)2) were immersed into a 5 g/l sodium borohydride (NaBH4) solution for 10 min. The activated fabrics were then collected for electroless copper plating.
The polyester fabrics activated with nickel were left in the electroless copper plating solution at 70 °C for 20 min. The plating bath consisted of 14 g/l of copper(II) sulfate pentahydrate (CuSO4·5H2O), 20 g/l of ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), 12 g/l of glyoxylic acid and 2 ml of potassium ferrocyanide. The pH value was adjusted by using sodium hydroxide (NaOH). Finally, the copper plated polyester fabrics were immediately rinsed with deionized water and dried in an oven at 60 °C.
For comparison purposes, electroless copper plating was also carried out on polyester fabric through conventional activation method. The activation of the cleaned fabric was performed by immersing the fabric into an aqueous solution of 5 g/l of nickel sulfate at room temperature for 10 min, followed by being treated in 5 g/l of an NaBH4 solution for 10 min. The subsequent processes of electroless copper plating are similar to those described above for the Ni(acac)2 activation process.
2.3 Characterization
The deposition rate (v: mg/(cm2 h)) of the electroless plating was determined by the gravimetrical method and expressed in terms of the weight gain per unit of plating area and time after the deposition. An analytical balance (Shimadzu BX300 m) with a precision of 0.1 mg was employed to weigh the fabrics before and after copper plating. The deposition rate was calculated with the following equation:
where v (mg/(cm2 h)) is the deposition rate; t (min) is the plating duration; Mt (mg) is the mass of the copper plated fabric for a length of time; M0 (mg) is the initial weight of the fabric and As (cm2) is the surface area of the fabric.
XPS was used for the surface analysis of the polyester fabrics before and after the activation process. XPS (PHI 5600, Physical Electronics) was performed by using an Al Kα source (14 kV and 350 W). The binding energy scale was calibrated to 285.0 eV for the main C (1s) peak. The surface morphology of the copper deposited the fabric was determined by SEM (JSM-6335F). The crystal structure of the copper plated fabrics was examined through XRD (Bruker D8 Discover, Cu Kα radiation and graphite filter at 40 kV and 40 mA). The surface resistance of the copper plated polyester fabrics was measured by using four-probe test system/Dual Electric Logging Four-Point Probe instrument (Guangzhou Four-point Probe Technology Company) was used to evaluate the surface resistance of the copper plated fabric.
The EMI shielding effectiveness (SE) is a measure of the ability of materials to attenuate electromagnetic waves, which refers to the logarithm of the ratio of the incident wave to the transmitted wave as shown in the following equation:
where P1 (E1) and P2 (E2) are the incident power (incident electric field) and the transmitted power (transmitted electric field), respectively [21].
The EMISE of the copper plated polyester fabrics was measured with an Agilent-E8363A vector network analyzer. The scanned frequency was 2–18 GHz. The attenuations under transmission and reflection were measured. The former is equivalent to the SE. The heat generation properties of the copper plated fabrics were investigated by using a thermocouple powered by a DC source with voltage values. The fabric sample with dimensions 5 cm × 5 cm was held between the two jaws. The temperature of the center of the plated fabrics was measured during heating with a thermocouple.
The copper plated polyester fabrics were washed in a detergent solution (0.37%) with 10 steel balls at 40 °C for 45 min in accordance with standard method AATCC 61-2003 (Colorfastness to Laundering), then rinsed in deionized water twice, and finally dried at 100 °C. The weight change of the fabric after laundering was used to assess the adhesive strength between the copper deposits and the polyester fabric. The weight loss of the copper coating before and after laundering was calculated after washing for 45 min.
3 Results and discussion
3.1 XPS analysis
Activation is a key process of electroless copper plating on fabric. Therefore, an XPS analysis of the Ni(acac)2 activated fabric was carried out to quantify the orientation distribution of the nickel catalysts on the polyester fibers. A wide scan XPS spectrum of the polyester fabric activated with Ni(acac)2 and narrow XPS spectrum of Ni 2p are shown in Fig. 1. The elements of C, O, Ni and Na are detected from the Ni(acac)2 activated polyester fabric (Fig. 1a). The elements of Ni and Na are attributed to the Ni(acac)2 and NaBH4, while C and O are both from the polyester fabric and Ni(acac)2. The presence of Ni indicates that Ni activation in scCO2 is successful. The peaks at 856 and 874.5 eV in Fig. 1b correspond to Ni 2p3/2 and Ni 2p1/2 of metallic nickel. These peaks indicate the presence of reduced nickel on the fabric. The XPS analysis confirms that the nickel catalyst is successfully infused into the polyester fibers in scCO2.
After the impregnation of Ni(acac)2 in scCO2, the polyester surface was covered with Ni(acac)2, which will provided the nucleation reactants for producing active sites. These Ni ions can be reduced by borohydride ions (BH4 −) to form active nuclei. The Ni particles serve to nucleate the copper deposition from the electroless plating bath.
The chemical reaction that takes place during activation can be written as follows:
After the activated polyester fabric is placed into the electroless copper plating solution, copper ions in the plating solution are reduced by glyoxylic acid and the copper particles are deposited on the nucleus. The copper coatings grow laterally and vertically, and finally, form a continuous and dense coating layer.
The reaction equation of electroless copper deposition is as follows:
3.2 Surface morphology
Figure 2 shows the SEM images of polyester fibers activated with nickel via the conventional process and with scCO2. When the conventional process is used, some of the nickel catalysts are found to be distributed on the polyester fibers (see Fig. 2a). However, in scCO2 process, a large amount of nickel catalysts are deposited onto the fibers and the fibers are covered with a dense and compact nickel catalysts film (see Fig. 2b). This result indicates that more nickel catalysts can be obtained via scCO2 process as opposed to the use of the conventional process.
Figure 3 shows the SEM images of the copper plated polyester fibers activated with nickel through conventional and scCO2 processes. Regardless of the method, the polyester fibers are compactly and uniformly covered with spherical copper particles after copper plating. The particle size of the copper coatings on the fibers activated via the conventional process ranges from 100 to 150 nm as shown in Fig. 3(a). However, the size of the copper particles via scCO2 process (Fig. 3b) ranges from 200 to 300 nm, which is obviously larger than the particle size obtained by using the conventional process. The polyester fibers activated with nickel can efficiently initiate copper deposition from the copper plating bath, which would result in fast crystal nucleation and growth. The migration and aggregation of copper particles are probably driven largely by the instability of the copper atoms due to their high surface free energy. Their aggregation would produce thermodynamically stable particles with larger sizes.
3.3 Deposition rate
The deposition rates of copper on the polyester fabrics activated with nickel via conventional and scCO2 processes are 8.61 and 15.12 mg/(cm2/h), respectively. The result shows that the rate of deposition of copper on the fibers activated via scCO2 process is higher than that via conventional process. Accordingly, the weight of the copper deposits on the fibers activated via scCO2 process is more than that via conventional process. This is because the nickel atoms on the polyester fabric act as privileged nucleation sites for copper plating. More nickel atoms on the fabric means more copper particles deposited onto the fabric. ScCO2 has low viscosity and high diffusivity. Therefore, the polyester fibers may be swollen with scCO2, and Ni(acac)2 is accordingly impregnated into the fibers. Nickel nuclei are not only deposited on the surface of the fibers but also infused into them. As the infused amount of nickel catalysts increases in the polyester fibers, the electroless copper plating reaction rate also increases.
The influence of scCO2 temperature on the deposition rate of copper coating on the fabric is shown in Fig. 4. It can be seen that the rate of deposition first increases with respect to the increase in temperature of scCO2. When the temperature of scCO2 is low, the reaction rate of nickel reduced by NaBH4 is low, which results in fewer nickel nuclei. Accordingly, the rate of plating of copper is low. As the temperature is increased, Ni2+ ions are increasing released gradually. Since the nickel ions reduced by NaBH4 are increased, there are more nickel nuclei, thus resulting in an increase in the rate of deposition of copper on the fabric.
3.4 Crystal structure
The XRD patterns of the copper plated polyester fabrics activated via conventional and scCO2 processes are shown in Fig. 5. The five strong characteristic peaks of the copper plated polyester fabrics at 2θ = 43.2°, 50.1°, 73.9°, 90.2° and 95.5° corresponded to the crystal faces of (111), (200), (220), (311) and (222) of copper, respectively. The XRD patterns identified by the PDF card of the JADE-SCAN software reveals that the deposited copper film exhibits characteristics of a face-centered cubic crystalline structure, which implies that the copper plated polyester fabrics have perfect conductivity. The copper oxide phase is not detected in the deposits. It is worth noting that the copper films formed under different conditions are very pure without any detectable impurities, such as CuO or Cu2O. As a result, the formation of copper oxide is inhibited.
Based on the XRD results, the crystal size of the copper coating was determined from the broadening of the diffraction peak by employing the Scherrer formula as expressed by the following equation
where t is the crystal size, λ is the X-ray wavelength that corresponds to Cu Kα radiation (0.154056 nm), θ is the diffraction angle, B is the full width half maximum of the diffraction peak at 2θ, and n is the Scherrer constant as 0.89 [22]. According to the Scherrer equation, the average crystal size of the copper particles on the polyester fibers activated via the conventional and scCO2 processes are 28.78 and 35.17 nm, respectively, with respect to the Cu (111) main peak.
3.5 Surface resistance
The surface resistance of the copper plated polyester fabrics activated with nickel via the conventional and scCO2 processes are 168 and 50 mΩ/sq, respectively. The result shows that the surface resistance of the copper plated fabrics activated via scCO2 process is superior to that activated via conventional process. The deposition rate of copper coating on the fibers activated via scCO2 process is higher than that via the conventional process. For the same deposition time, the deposition weight of the copper coating on the fibers via scCO2 process is more than that via conventional process. A higher deposition weight results in lower surface resistance. This may be because more copper particles are deposited onto the polyester fabric with scCO2 in the pretreatment of the polyester fabric. Therefore, the use of scCO2 as a pretreatment can provide better electrical conductivity.
The surface resistances of the copper plated fabrics with different temperature of scCO2 are shown in Fig. 6. It can be observed that the surface resistance of the copper deposits first decreases and then increases with respect to the increase in temperature of scCO2. The decrease in surface resistance is mostly attributed to the increase in the weight of the copper deposition, particle size and crystallinity. The surface resistance is high due to less copper deposition at low temperatures of scCO2. The surface resistance reaches 50 mΩ/sq at 90 °C because of a higher reaction rate and large copper particle size. However, the reaction rate is too high at higher temperatures, thus causing lattice defects in the crystalline.
3.6 EMI SE of copper plated polyester fabric
The EMI SE results of the copper plated fabrics activated with Ni(acac)2 via the conventional and scCO2 processes at different temperature of scCO2 are shown in Fig. 7. The EMI SE of the copper plated polyester fabrics via conventional process is more than 40 dB at frequencies from 2 to 18 GHz. It is obvious that the EMI SE of the copper plated polyester fabric activated via scCO2 process at 60 and 90 °C ranges from 45 to 55 dB and 50 to 60 dB respectively at frequencies of 2–18 GHz. The EMI SE of copper plated polyester fabric activated with Ni(acac)2 at 90 °C via scCO2 process is improved due to acceleration of copper deposition which resulted from an increase in the amount of nickel nuclei for electroless copper plating. This indicates that temperature of scCO2 obviously affects the EMI SE of the copper plated polyester fabric.
The copper plated polyester fabric activated with Ni(acac)2 via scCO2 process effectively attenuates the penetrability of electromagnetic waves. Hence, this fabric has practical use for many EMI shielding applications, especially those for high frequency electromagnetic fields.
3.7 Heat generation
The temperatures of the copper plated fabrics over a period of 30 s at different DC voltages are shown in Table 1. The initial temperature of the fabrics is room temperature. However, it is notable that the temperature of copper plated fabric can be maintained from 28.1 to 37.4 °C with only 4 V. The result shows that the copper plated fabrics can be employed to generate heat. However, there is more heat generated via scCO2 process as opposed to the use of the conventional process due to the greater electrical conductivity provided by larger and more copper particles. In addition, after the steady state is reached, the fabric temperature does not change when the fabric is charged over a long time under a constant voltage, which indicates that the fabric resistance remains unchanged and the conductive coating is stable during the heating experiments. The heat stability of the copper plated fabric activated via scCO2 process excels that when the conventional process is used. Thus, copper plated fabrics can have various applications, such as flexible heating pads and smart wearable clothing.
3.8 Water contact angle of copper plated polyester fabric
Wettability is usually evaluated by measuring the water contact angle. Figure 8 shows the water droplets on the original, Ni(acac)2 activated and copper plated polyester fabrics activated via conventional and scCO2 processes. The original polyester fabric can be partly wetted by water due to the low density of the fabric. Therefore, the water contact angle of original polyester fabric is 81.06° (Fig. 8a). However, the contact angle of the Ni(acac)2 activated polyester fabric is 10.05° (Fig. 8b). The fabric becomes hydrophobic with a water contact angle of 110.80° after the polyester fabric activated via conventional process is plated with copper particles (Fig. 8c). This indicates that the deposition of copper in the spaces between yarns partly reduces the number of void areas in the fabric and reduces the water penetration of the copper plated fabric. However, the water contact angle of the copper plated polyester fabric activated via scCO2 process is 136.17° as shown in Fig. 8d. This indicates that copper plated polyester fabric activated via scCO2 process is hydrophobic due to the increase of its surface roughness and reduction of void areas. The air that is trapped on a rough surface with protuberances and cavities on the polyester fabric can significantly enhance the surface hydrophobilicity. The water contact angle is usually defined by Cassie’s equation as follows:
where θ* is the water contact angle on a rough surface; θ is the water contact angle on a flat surface; and φs is the fraction of the solid surface in contact with water droplets.
The difference in the hydrophobilicity of a surface is due to the difference in surface roughness. In Cassie’s equation, cosθ* is always less than cosθ because φs is less than or equal to 1; that is, cosθ* is less than or equal to cosθ, regardless of θ.
From the SEM images in Fig. 3, it can be seen that the surface of the copper plated fabric activated via scCO2 process is rougher than that with the use of the conventional process. Therefore, larger water contact angles can be found in copper plated polyester fabric via scCO2 process. The results confirm that activation in scCO2 has a profound effect on hydrophobic properties. The increase of the contact angle on the surface of polyester fabrics activated via scCO2 process is due to the increase in its surface roughness.
3.9 Thermal behaviors
The thermal behavior of the original, copper plated polyester fabrics activated via conventional and scCO2 processes were investigated and the TGA curves are presented in Fig. 9. For all of the samples, the curves are similar, and the result shows that the two different activation processes have little impact on the thermal stability of polyester fabric. There is significant weight loss of original fabric, as well as the copper plated polyester fabrics activated via the conventional and scCO2 processes when the temperature is 300, 380 and 430 °C respectively due to the degradation of the fabric. The heat resistance of polyester fabric is improved after copper plating, which instills better flame retardancy. The residual of original, copper plated polyester fabrics activated via conventional and scCO2 processes are close to 3, 40 and 62%, respectively. This indicates that the polyester fabric is almost decomposed. The excess residue available in the plated fabric is due to the presence of copper particles. The contents of copper particles on the polyester fabric activated via the conventional and scCO2 processes are 40 and 62%, respectively. In addition, the thermal decomposition residues of copper plated polyester fabric activated via scCO2 process is more than that of the fabric that was activated via conventional process because there are more copper particles deposited onto the fabric.
3.10 Adhesive strength
The adhesion of metal to fabric is one of the most important concerns in electroless plating. Therefore, the colorfastness to laundering test was employed to evaluate the adhesion of the copper films onto the fabric, and repeated five times for each specimen. For comparison, the test was also carried out with the copper plated polyester fabric activated via the conventional process.
The weight loss and surface resistance of the copper plated fabrics after 5 cycles of home washing are shown in Table 1. There is weight loss of the copper deposits after washing. It can be observed that there is less weight loss and increase of surface resistance of the copper plated fabric via scCO2 process as opposed to that via the conventional process after five cycles of washing. The adhesive strength between the copper deposits and the polyester fabric is remarkably improved because scCO2 is both as a solvent for infusing nickel activators into fibers and a plasticizer that softens the surface of the fiber substrates and increases the adhesion between the copper and the fiber substrate. Thus, the use of scCO2 process is validated as a means of improving the adhesion of copper particles onto a polyester fiber.
4 Conclusion
A novel palladium-free activation process has been designed in which polyester fabric is pretreated with Ni(acac)2 via scCO2 process prior to electroless copper plating. The results indicate that there is much more copper deposited onto polyester fabric via scCO2 process than that via conventional process. The copper particles plated on the polyester fibers via scCO2 activation are spherical and larger in size than those obtained through the conventional process. The surface resistance and EMISE of copper plated polyester fabrics activated via scCO2 process is 50 mΩ/sq and 50–60 dB at frequencies from 2 to 18 GHz, respectively. The water contact angle and temperature of the fabric with a voltage of 4 V are 136.17 and 37.4 °C, respectively. These results suggest that the activation via scCO2 process prior to electroless copper plating provides polyester fabric with excellent electrical conductivity, EMI SE and heat generation. In addition, the copper particles are uniformly deposited on the polyester fibers and strongly adhere to the polyester fibers.
References
F. Tian, E.A. Decker, D.J. McClements, J.M. Goddard, Influence of non-migratory metal-chelating active packaging film on food quality: impact on physical and chemical stability of emulsions. Food Chem. 151, 257–265 (2014)
V. Safarova, J. Militky, Multifunctional metal composite textile shields against electromagnetic radiation-effect of various parameters on electromagnetic shielding effectiveness. Polym. Compos. 38, 309–323 (2017)
M. Rai, S.D. Deshmukh, A.P. Ingle, I.R. Gupta, M. Galdiero, S. Galdiero, Metal nanoparticles: the protective nanoshield against virus infection. Crit. Rev. Microbiol. 42, 46–56 (2016)
Y.H. Chen, C.Y. Huang, F.D. Lai, M.L. Roan, K.N. Chen, J.T. Yeh, Electroless deposition of the copper sulfide coating on polyacrylonitrile with a chelating agent of triethanolamine and its EMI Shielding Effectiveness. Thin Solid Films 517, 4984–4988 (2009)
Y.H. Chen, C.Y. Huang, M.L. Roan, F.D. Lai, K.N. Chen, J.T. Yeh, The copper sulfide coating on polyacrylonitrile with a chelating agent of ethylenediaminetetraacetic acid by an electroless deposition method and its EMI shielding effectiveness. J. Appl. Polym. Sci. 115, 570–578 (2010)
E.S. Ji, H.G. Cha, C.W. Kim, D.I. Kang, Y.S. Kang, Copper plating on the polyimide film by electroless plating techniques for EMI shielding. J. Nanosci. Nanotechnol. 9, 7065–7070 (2009)
H. Zhang, L.P. Shen, J. Chang, Comparative study of electroless Ni–P, Cu, Ag, and Cu–Ag plating on polyamide fabrics. J Ind. Text. 41, 25–40 (2011)
H.Y. Chen, Y. Tai, C.J. Xu, Fabrication of copper-coated glass fabric composites through electroless plating process. J. Mater. Sci. 28, 798–802 (2017)
H.S. Hwang, T. Yoon, J. Jang, J.J. Kim, J.H. Ryu, S.M. Oh, Carbon fabric as a current collector for electroless-plated Cu6Sn5 negative electrode for lithium-ion batteries. J. Alloy Compd. 692, 583–588 (2017)
S.M. Kim, I.Y. Kim, H.R. Kim, Production of electromagnetic shielding fabrics by optimization of electroless silver plating conditions for PET fabrics. J. Text. Inst. 108, 1065–1073 (2017)
G.H. Zheng, J.H. Ren, X.G. Zhang, R.H. Guo, F.L. Ji, Research of the electroless copper plating on wool fabrics. Microsyst. Technol. 22, 929–934 (2016)
Y.X. Lu, Q. Liang, W.L. Li, Fabrication of copper/modal fabric composites through electroless plating process for electromagnetic interference shielding. Mater. Chem. Phys. 140, 553–558 (2013)
W.F. Qin, R.H. Guo, Influence of K 4fe (Cn) 6 on electroless copper plated polyester fabric using glyoxylic acid as a reducing agent. Tekst Konfeksiyon 25, 319–322 (2015)
S. Tengsuwan, M. Ohshima, Environmentally benign electroless nickel plating using supercritical carbon-dioxide on hydrophilically modified acrylonitrile-butadiene-styrene. Appl. Surf. Sci. 311, 189–200 (2014)
W.X. Ma, C.A. Zhao, S. Okubayashi, I. Tabata, K. Hisada, T. Hori, A novel method of modifying poly(ethylene terephthalate) fabric using supercritical carbon dioxide. J. Appl. Polym. Sci. 117, 1897–1907 (2010)
S.P. Nalawade, F. Picchioni, L.P.B.M. Janssen, Supercritical carbon dioxide as a green solvent for processing polymer melts: processing aspects and applications. Prog. Polym. Sci. 31, 19–43 (2006)
S. Tengsuwan, M. Ohshima, Electroless nickel plating on polypropylene via hydrophilic modification and supercritical carbon dioxide Pd-complex infusion. J. Supercrit. Fluid 69, 117–123 (2012)
T. Hori, A. Kongdee, Dyeing of PET/co-PP composite fibers using supercritical carbon dioxide. Dyes Pigments 105, 163–166 (2014)
Y. Iwai, S. Sameshima, S. Yonezawa, S. Katayama, Fabrication of conductive cotton by electroless plating method with supercritical carbon dioxide. J. Supercrit. Fluid 100, 46–51 (2015)
O. Pascu, L. Marciasini, S. Marre, M. Vaultier, M. Pucheault, C. Aymonier, Continuous coflow synthesis of hybrid palladium nanocrystals as catalysts for borylation reaction. Nanoscale 5, 12425–12431 (2013)
R. Guo, S. Jiang, C. Yuen, M. Ng, An alternative process for electroless copper plating on polyester fabric. J. Mater. Sci. 20, 33–38 (2009)
C. Charton, M. Fahland, Growth of Ag films on PET deposited by magnetron sputtering. Vacuum 68, 65–73 (2002)
Acknowledgements
This work was financially supported by The National Natural Science Foundation of China (No. 51203099).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, R., Jing, X., Peng, L. et al. Nickel-catalyzed deposition of Cu film on PET fabric with supercritical fluid. J Mater Sci: Mater Electron 28, 16618–16626 (2017). https://doi.org/10.1007/s10854-017-7572-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7572-4