Abstract
Ceramic matrix containing zirconolite, hollandite, and perovskite phases is proposed as a potential host for HLW immobilization. Hollandite phase principally immobilizes Cs, while perovskite phase mainly immobilizes Sr. In this study, hollandite–perovskite composite ceramics are considered as a specialized waste form for immobilizing the separated Cs and Sr from HLW streams and synthesized by a solid-state reaction method at 1300 °C for 5 h. The phase compositions of the synthesized composites were characterized by XRD and BSE. The XRD results indicated that the as-prepared ceramics are composed of tetragonal hollandite Ba0.8Cs0.4Al2Ti6O16, cubic perovskite SrTiO3, alongside a lesser amount of TiO2. The BSE—EDX results confirm that Cs partitions into the hollandite matrix, while Sr incorporates into perovskite host with homogenous distribution. In addition, aqueous durability testing was carried out using the MCC-1 static leach test method. The normalized release rates of Cs and Sr in HP-3 sample (i.e., 75 wt% Ba0.8Cs0.4Al2Ti6O16 + 25 wt% SrTiO3) were < 10−2 g·m−2·d−1 after 42 days, exhibiting excellent chemical durability. These results indicate that the hollandite–perovskite ceramic matrix could be considered as a customized host matrix for immobilization of the separated Cs and Sr from HLW streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disposal of radioactive wastes, especially high-level nuclear wastes (HLW) produced during the reprocessing of spent nuclear fuels [1, 2], is still a challenging task because of their high radiotoxicity [3,4,5,6]. Until now, borosilicate glass is the only waste form applied at the industrial scale [7, 8]. Due to superior stable nature, ceramic waste form materials may improve the long-term aqueous performance in the disposal environment, relative to vitrified matrices [9, 10]. In order to properly dispose of the radioactive hazards and diminish their effects on the environment, crystalline ceramics have been regarded as a potential nuclear host to immobilize HLW [11,12,13,14]. On the atomic-scale, the radioactive waste elements are usually incorporated into the lattices structure of Synroc mineral phases, which would provide a more secure immobilization barrier compared with that in nuclear glasses. Previous results indicated that the normalized release rates of Cs, Sr, Ca and U in ceramic waste form are ~ 7.8 × 10–3, 7.0 × 10−4, 8.0 × 10−3, < 10−4 g·m−2·d−1 respectively, which is 2 ~ 3 orders of magnitude lower than that of 1.03, 7.5 × 10−2, 6.8 × 10−2, 0.1 g·m−2·d−1 in borosilicate glass [15,16,17]. In addition, 238Pu-doped Synroc-C remains primary crystalline matrix for cumulative radiation doses of 1019 alpha/g [18], illustrating ceramic waste form is a satisfactory host for HLW.
Cesium (Cs) and strontium (Sr) are two of the major fission products and commonly exist in the nuclear waste stream from the reprocessing of spent fuels and primarily responsible for the heat generation in the storage system during the first few centuries [19, 20]. As two major concerned fission products for disposal of HLW, separation of Cs and Sr from spent nuclear fuel will reduce the nuclear waste volumes and radioactivity of HLW, which can simplify waste-handling operations and reduce the thermal load of the HLW storage [21, 22]. Due to high toxicity and solubility, the separated Cs and Sr must be immobilized in a robust host for final disposal. Previous results demonstrated that hollandite—type are of great interest to act as a host for Cs, while perovskite matrices are suitable to host Sr [23,24,25]. The general formula of hollandite is AxByC8-yO16 (x ≤ 2), A-sites are occupied by the large ions (i.e., Cs+, Ba2+, Rb+), while small cations (i.e., Mg2+, Al3+, Ti4+, Fe3+ and Sb5+) are located at the B and C-site. In AxByC8-yO16, the hollandite structure comprises corner and edge-sharing BO6 and CO6 octahedra forming tunnels along the c-axis, and the large A-site ions are located in these tunnels [26]. In perovskite (ABO3), BO6 octahedra share corners to form a 3D framework with large central cavities, the A cations are located in these cavities [27]. The naturally mineral of perovskite is CaTiO3, which is easy to incorporate Sr in the perovskite structure due to the similar ionic and the similar chemistry of Ca and Sr. Importantly, in SYNROC-C, hollandite and perovskite phases are specifically targeted to accommodate Cs and Sr, respectively [28]. Thus, hollandite–perovskite composite ceramics are expected to be an excellent host matrix for final disposal of Cs and Sr.
Motivated by merits of hollandite-type ceramics and perovskite matrices above, we developed a hollandite–perovskite ceramic waste form for immobilization of Cs and Sr simultaneously. The Ba0.8Cs0.4Al2Ti6O16—SrTiO3 assemblage was synthesized in our work by a solid-state reaction method. The phase composition, microstructure and chemical durability of the as-prepared ceramic waste form were systemically investigated.
Experimental
Synthesis of hollandite–perovskite ceramics
The hollandite–perovskite composite ceramics were designed and synthesized to immobilize Cs and Sr. The nominal phase compositions are: 85 wt%, 80 wt%, 75 wt%, 70 wt%, 65 wt%, and 60 wt% for Cs0.4Ba0.8Al2Ti6O16, and 15 wt%, 20 wt%, 25 wt%, 30 wt%, 35 wt% and 40 wt% for SrTiO3 (named them as HP-1, HP-2, HP-3, HP-4, HP-5, and HP-6). In a typical process, Analytical Reagent (AR) starting materials of Cs2CO3 (99% purity, Aladdin Co. Ltd.), BaCO3 (99% purity, Aladdin Co. Ltd.), SrCO3 (99% purity, Aladdin Co. Ltd.), Al2O3 (98% purity, Aladdin Co. Ltd.) and TiO2 (99% purity, mixture of rutile and anatase, Aladdin Co. Ltd.) are used to prepare samples. All initial powders are pre-heated at 120 °C for 2 h in order to remove adsorbed water. The dried powders were prepared according to Table 1 and sufficiently homogenized by the agate mortar and pestle in ethyl alcohol media for 2 h. The homogenized mixtures were dried and pressed into pellets (12 mm in diameter and 3 mm in thickness) at a pressure of 12 MPa. To explore an optimum sintering temperature, a representative HP-3 sample (i.e., 75 wt% Ba0.8Cs0.4Al2Ti6O16 + 25 wt% SrTiO3) was chosen and sintered at 1200–1350 °C for 5 h in ambient atmosphere, with heating rate of 10 °C/min. After that, the compacted HP-1 ~ 6 pellets were sintered at the optimum sintering temperature.
Characterization
Simultaneous thermogravimetry and differential scanning calorimetry (TG-DSC, SDT Q600) was used to investigate the thermal behavior of hollandite–perovskite ceramic from room temperature to 1350 °C with a heating rate of 10 °C/min and 100 mL/min air flow. The crystalline phases of the sintered ceramics were characterized by X-ray diffraction (XRD, X'Per PRO, Netherlands) with Cu Kα radiation (λ = 1.5406 Å). The data were collected at 2 θ = 10 ~ 90° with step size of 0.02°. The General Structure Analysis System (GSAS) program was employed to obtain the lattice parameters of targeted hollandite and perovskite matrices in the synthesized materials [29]. The refinements proceeded as follows: the initial structural model for the Ba0.8Cs0.4Al2Ti6O16, SrTiO3 and TiO2 Rutile were taken from the crystallographic data of Cs0.187Ba0.965Al2.115Ti5.885O16 (JCPDS card no. 78–0018, space group I4/m), SrTiO3 (JCPDS card no. 35–0734, space group Pm\(\overline{3}\)m) and TiO2 (JCPDS card no. 73–1765, space group P42/mnm). At the beginning of refinements, the scale factor and background were firstly converged, then the lattice parameters and phase fraction were added and refined. Final, the peak profiles were fitted to pseudo-Voigt convolution functions. On convergence of the preceding parameters, the atomic coordinates and atomic isotropic temperature factors were also refined. The sintered HP-3 sample, as a representative of the sintered pellets was selected to investigate phase distribution and aqueous durability of hollandite–perovskite ceramics. The HP-3 sample was polished by metallographic sandpaper to a roughness of ~ 1 μm. The phase distribution of the polished HP-3 sample was studied using backscattering electron (BSE, Ultra55, Zeiss). Energy-dispersive X-ray spectroscopy (EDX, IE450X-Max80, Oxford) attached to the BSE equipment was used to collected elemental maps from multiphase regions and identify chemical composition of the targeted hollandite and perovskite matrices.
A leaching test was carried out using the static leach test (Materials Characterization Center, MCC-1) method [30]. The sample HP-3 was suspended in the closed Teflon container with deionized water. The sealed vessels were then placed in an oven maintained at 90 ± 2 °C for a period of 1–42 days. The concentrations (Ci) of Cs and Sr in leachate were analyzed by inductively coupled plasma mass spectrometry (ICP-Mass, Agilent 7700x, Agilent, USA). The normalized leaching rates (NLi, g·m−2·d−1) of Cs and Sr were calculated as the following equation:
where Ci (g·m−3) is the concentration of element i = (Cs and Sr) in the leachate, V (m3) is the volume of the leachate, SA (m2) is the surface area of leached samples, fi (wt%) is the mass fraction of element i in the leached ceramics and tn (days) is the leaching time.
Results and discussion
Phase formation and crystalline structure
Figure 1 shows the TG-DSC curves of the precursor powders used in the synthesis of HP-3 ceramic sample. As shown in the TG-DSC graph, four weight loss stages are distinctly observed from room temperature to 1250 °C. For the first stage from room temperature to 400 °C, a mass loss of 1.8% is ascribed to the dehydration physisorbed and chemisorbed water in the precursor powders [31], corresponding to endothermic peak at 70.2 °C in the DSC curve. The second stage with 2.4% mass loss between 400–570 °C could be due to the decomposition of Cs2CO3 to Cs2O, homologizing the endothermic peak at 534.3 °C in the DSC curve [32]. With regard to the third stage between 570 and 840 °C, a weight loss of 1.6% was observed in the TG curve, accompanied by an endothermic peak at 817.5 °C in the DSC curve, which may be assigned to the decomposition of SrCO3 [33]. The last stage with 4.6% mass loss in the range of 840–1020 °C and the endothermic peak at 935.7 °C may be attributed to the decomposition of BaCO3 [34]. Notably, the mass is almost unchanged at 1020–1250 °C, and the DSC curve displays a broad exothermic peak due to the crystallization of hollandite and perovskite phases. This result indicates that the targeted hollandite–perovskite waste form could be synthesized above 1020 °C.
It is well known that elemental Cs and most cesium compounds are volatile at high temperature (> 900 °C). An elevated sintering temperature could lead to a severe Cs loss. As shown in Fig. 1, ~ 0.3% of weight loss is observed at 1250 ~ 1350 °C, indicating a poor Cs retention in sintered sample at elevated temperatures. To explore an optimum sintering temperature, HP-3 powder precursors were sintered at 1200, 1250, 1300 and 1350 °C for 5 h, respectively.
Figure 2a presents the XRD patterns for HP-3 samples sintered at different temperatures (1200, 1250, 1300 and 1350 °C) for 5 h. It is found that the hollandite–perovskite composite ceramics are formed at 1200 °C with small amount of unreacted TiO2 and the BaTi5O11 metastable intermediate phase [35]. With increasing sintering temperature, the metastable BaTi5O11 phase disappears, indicating an increasing sintering temperature plays a significant role in improving the phase purity of the targeted hollandite–perovskite ceramics. However, after sintering at 1350 °C, TiO2 phase is still observable in the synthesized sample. The TiO2 probably originates from excess Ti, due to Cs volatilization at the high temperature [36, 37]. It is worth noting that the peak intensity of TiO2 phase in the XRD patterns of 1300 °C sintered sample is weaker than that of 1250 and 1350 °C, suggesting a lower TiO2 content which can be verified by XRD refined compositions. As shown in Fig. 2b-d, the calculated phase compositions demonstrate that the weight fraction of TiO2 in 1300 °C sintered sample is lower that of 1250 and 1350 °C synthesized samples. This result indicates that the hollandite–perovskite composite ceramics with high phase purity can be considered to fabricating at 1300 °C for 5 h; this was thus selected.
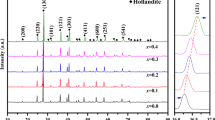
XRD patterns of the sintered hollandite–perovskite ceramics at 1300 °C for 5 h are displayed in Fig. 3. As observed in Fig. 3, all samples are major hollandite and perovskite phases with little of TiO2, suggesting the desired matrices have been formed.
To further investigate the phase compositions of as-prepared samples at 1300 °C, XRD data are analyzed by the Rietveld method using the GSAS program, the calculated phase compositions and unit-cell parameters are listed in Table 2. From Table 2, the refined results further illuminate that all samples are composed of hollandite, perovskite and TiO2. In addition, for the targeted hollandite and perovskite matrices, the refined structural parameters fit well with the tetragonal hollandite (a = b ~ 9.99 Å, c ~ 2.92 Å) and cubic perovskite (a = b = c ~ 3.91 Å) phases. This is agreement with powder diffraction data for Cs0.187Ba0.965Al2.115Ti5.885O16 (PDF# 78–0018) and SrTiO3 (PDF# 35–0734). Combined with TG-DSC analysis, the schematic of solid-state method for hollandite–perovskite ceramic waste form synthesis is summarized in Fig. 4.
Phase distribution and chemical composition
The phase distribution and chemical composition are identified using BSE-EDX analysis. Figure 5 shows the BSE contrast images and EDX analysis of polished HP-3 ceramic sample. It can be found in Fig. 5a that three contrast discrepancies are clearly observed, indicating these three phases coexist in the as-prepared composite. In addition, these three crystalline phases observed in the BSE image are verified using EDX elemental mapping analysis (Fig. 5b–f). The EDX element mapping collected on different matrices reveals that the “light grey” region is rich in Sr and Ti, “grey” region is rich in Cs, Ba, Al and Ti, while “dark grey” area is only rich in Ti. According to the result of the EDX analysis, it can be confirmed that the light grey, grey and dark grey phases correspond to perovskite, hollandite and TiO2 matrices respectively, in agreement with above XRD results (Fig. 3).
To investigate the chemical compositions of the targeted hollandite and perovskite matrices in the mixture, the EDX spectra of the selected regions (labeled as “A” and “B” in Fig. 5a) are present in Fig. 5g, h. As seen in EDX spectra, Cs L, Ba L, Ti K, Al K and O K peaks exist in “A” zone while the “B” area contains the peaks of Sr L, Ti K and O K, further confirming the “light grey” matrix is perovskite while the “grey” phase is hollandite. Intriguingly, the calculated formula of perovskite phase in the sample is Sr0.96Ti1.02O3 (Fig. 5h), which is perfectly consistent with the original designed constituent SrTiO3. However, in comparison with Sr-bearing perovskite, Cs- bearing hollandite exhibits an obvious deviation from designed stoichiometry (i.e., Cs0.4Ba0.8Al2Ti6O16), only ~ 67.5% of the targeted Cs concentration is retained within the prepared hollandite matrix (Fig. 5g). The Cs loss is due to Cs vaporization during sintering, and supported by previous results [38, 39]. It is worth to mention the previous investigations show the optimum sintering temperature for the (Cs, Ba)-hollandite ceramics is around 1250 °C using high temperature solid-state method [40, 41], thus it is difficulty to improve the Cs retention in our work using the same method. Accordingly, the hot pressed sintering method will be further explored in our next work due to its predominant property with shorter sintering period and lower sintering temperature [42], which may give rise to reduction of cesium volatilization.
Chemical durability
To evaluate the chemical durability of hollandite–perovskite ceramic, MCC-1 is performed on the selected HP-3 composition. The normalized release rates of Cs and Sr in HP-3 sample are measured over a 42-day period, and the results are shown in Fig. 6. It can be seen in Fig. 6 that the normalized leaching rates of Cs and Sr decrease rapidly during the 14-day test period and slowly decrease with increasing time, then remain nearly constant after 14 days. This result indicates that the leaching behavior of Cs and Sr in hollandite–perovskite ceramics can be explained by the interfacial dissolution-re-precipitation mechanism [43, 44]. It should be noted that, after 42 days leaching, the calculated NLCs and NLSr in the HP-3 sample (5.2 wt.% cesium + 16.9 wt.% strontium waste loading on an oxide basis) are respectively 7.83 × 10–3 g·m−2·d−1 and 4.32 × 10–5 g·m−2·d−1, which is lower than that of 10–1 ~ 10 g·m−2·d−1 in the borosilicate glass waste form with 1.09 wt.% cesium oxide and 0.40 wt.% strontium oxide loading [15, 30]. Moreover, the obtained data are also slightly lower than the reported values of ~ 10–2 g·m−2·d−1 for Cs and Sr in synroc-C [45], exhibiting an excellent leaching resistance to Cs and Sr.
Figure 7 presents SEM–EDS images and XRD patterns of the prepared sample before and after leaching. It can be seen from Fig. 7a that the pre-leaching sample possesses a smooth and clean surface morphology, while some floccules are observed on surface of post-leaching sample after leaching 42 days (Fig. 7b). The observed floccules could be ascribed to the formation of precipitates with respect to the released ionic species [46]. However, the precipitate phases fail to be determined by EDS and XRD, owing to their contents below the instrumental detection limit [10, 47]. As observed in Fig. 7c, the elemental mapping analysis reveals the homogeneous distribution of Cs Ba, Al, and Ti in the “H” zone and the Sr and Ti are distributed uniformly throughout the “P” area. Importantly, the EDS analysis indicates that the chemical compositions of hollandite matrix is well agreement between the pre-leaching and post-leaching HP-3 samples (Figs. 5g and 7d), as well as perovskite host (Figs. 5h and Fig. 7e ). Furthermore, the additional peaks are not observed in the post-leaching sample in the XRD patterns, in accordance with the previous results [48]. It confirms that the aqueous corrosion does not induce substantial structural changes of the Cs- and Sr-bearing hosts. Therefore, hollandite–perovskite ceramics are considered as a promising waste form for safety immobilization of the separated Cs and Sr from HLW streams.
Conclusions
In the present study, a hollandite–perovskite ceramic matrix is considered as a promising host matrix for Cs and Sr immobilization. The phase composition, crystal structure and chemical durability of the synthesized composites are investigated. The results show that hollandite and perovskite are the major phases in the all samples along with a small amount of TiO2. Moreover, Cs-bearing hollandite matrix shows a tetragonal structure (I4/m) while Sr-bearing perovskite exhibits a cubic structure (Pm\(\overline{3}\)m). The 42 days normalized leaching rates of Cs and Sr were measured to be 7.83 × 10–3 g·m−2·d−1 and 4.32 × 10–5 g·m−2·d−1, respectively. Moreover, the aqueous corrosion does not induce substantial structural changes of Cs/Sr-bearing hosts. These results suggest that the hollandite–perovskite waste form is a promising candidate to safely immobilize the separated Cs and Sr waste stream from HLW.
References
Ewing RC, Whittleston RA, Yardley BW (2016) Geological disposal of nuclear waste: a primer. Elements 12:233–237. https://doi.org/10.2113/gselements.12.4.233
Dhara A, Mishra RK, Shukla R, Valsala TP, Sudarsan V, Tyagi AK, Kaushik CP (2016) A comparative study on the structural aspects of sodium borosilicate glasses and barium borosilicate glasses: effect of Al2O3 addition. J Non Cryst Solids 447:283–289. https://doi.org/10.1016/j.jnoncrysol.2016.04.040
Goel A, McCloy JS, Pokorny R, Kruger AA (2019) Challenges with vitrification of hanford high-level waste (HLW) to borosilicate glass: an overview. J Non Cryst Solids X 4:100033 (1-19). https://doi.org/10.1016/j.nocx.2019.100033
Salvatores M, Palmiotti G (2011) Radioactive waste partitioning and transmutation within advanced fuel cycles: achievements and challenges. Prog Part Nucl Phys 66:144–166. https://doi.org/10.1016/j.ppnp.2010.10.001
McMaster SA, Ram R, Faris N, Pownceby MI (2018) Radionuclide disposal using the pyrochlore supergroup of minerals as a host matrix: a review. J Hazard Mater 360:257–269. https://doi.org/10.1016/j.jhazmat.2018.08.037
Beswick AJ, Gibb FGF, Travis KP (2014) Deep borehole disposal of nuclear waste: engineering challenges. Proc Inst Civil Eng Energy 167:47–66. https://doi.org/10.1680/ener.13.00016
Ojovan MI, Lee WE (2011) Glassy wasteforms for nuclear waste immobilization. Metall Mater Trans A 42:837–851. https://doi.org/10.1007/s11661-010-0525-7
Gin S, Jollivet P, Tribet M, Peuget S, Schuller S (2017) Radionuclides containment in nuclear glasses: an overview. Radiochim Acta 105:927–959. https://doi.org/10.1515/ract-2016-2658
Ringwood AE, Oversby VM, Kesson SE, Sinclair W, Ware N, Hibberson W, Major A (1981) Immobilization of high-level nuclear reactor wastes in synroc: a current appraisal. Nucl Chem Waste Manag 2:287–305. https://doi.org/10.1016/0191-815X(81)90055-3
Meng C, Li W, Ren C, Zhao J (2020) Structure and chemical durability studies of powellite ceramics Ca1−xLix/2 Gdx/2MoO4 (0 ≤ x ≤ 1) for radioactive waste storage. J Mater Sci 55:2741–2749. https://doi.org/10.1007/s10853-019-04223-y
Hsieh YH, Rushton MJD, Fossati PCM, Lee WE (2020) Thermal footprint of a geological disposal facility containing EURO-GANEX wasteforms. Prog Nucl Energy 118:103065. https://doi.org/10.1016/j.pnucene.2019.103065
Wang L, Liang T (2012) Ceramics for high level radioactive waste solidification. J Adv Ceram 1:194–203. https://doi.org/10.1007/s40145-012-0019-8
Ravikumar R, Gopal B, Jena H (2020) Fabrication, chemical and thermal stability studies of crystalline ceramic wasteform based on oxyapatite phosphate host LaSr4(PO4)3O for high level nuclear waste immobilization. J Hazard Mater 394:122552. https://doi.org/10.1016/j.jhazmat.2020.122552
Orlova AI, Ojovan MI (2019) Ceramic mineral waste-forms for nuclear waste immobilization. Materials 12:2638. https://doi.org/10.3390/ma12162638
Stefanovsky SV, Yudintsev SV, Gieré R, Lumpkin GR (2004) Nuclear waste forms. Geol Soc Lond Spec Publ 236:37–63. https://doi.org/10.1144/GSL.SP.2004.236.01.04
Luo S, Li L, Tang B, Wang D (1998) Synroc immobilization of high level waste (HLW) bearing a high content of sodium. Waste Manag 18:55–59. https://doi.org/10.1016/S0956-053X(97)00019-6
Newkirk H, Ryerson F, Coles D, Hoenig C, Rozsa R, Rossington C, Bazan F, Tewhey J (1980). Phase equilibria, leaching characteristics and ceramic processing of SYNROC D formulations for US defense wastes (No. UCRL-85483; CONF-801124–45). California Univ, Livermore (USA) Lawrence Livermore National Lab
Hambley MJ, Dumbill S, Maddrell ER, Scales CR (2008) Characterisation of 20 year old 238Pu-doped Synroc C (conference paper). Mater Res Soc Symp Proc 1107:373–380. https://doi.org/10.1557/PROC-1107-373
Chao X, Wang J, Chen J (2012) Solvent extraction of strontium and cesium: a review of recent progress. Solvent Extr Ion Exc 30:623–650. https://doi.org/10.1080/07366299.2012.700579
Sengupta P, Sanwal J, Mathi P, Mondal JA, Mahadik P, Dudwadkar N, Gandhi PM (2017) Sorption of Cs and Sr radionuclides within natural carbonates. J Radioanal Nucl Chem 312:19–28. https://doi.org/10.1007/s10967-017-5206-1
Dozol JF, Dozol M, Macias RM (2000) Extraction of strontium and cesium by dicarbollides, crown ethers and functionalized calixarenes. J Incl Phenom Macro 38:1–22. https://doi.org/10.1023/A:1008145814521
Mimura H, Akiba K, Igarshi H (1993) Removal of heat-generating nuclides from high-level liquid wastes through mixed zeolite columns. J Nucl Sci Technol 30:239–247. https://doi.org/10.1080/18811248.1993.9734476
Tumurugoti P, Clark BM, Edwards DJ, Amoroso J, Sundaram SK (2017) Cesium incorporation in hollandite-rich multiphasic ceramic waste forms. J Solid State Chem 246:107–112. https://doi.org/10.1016/j.jssc.2016.11.007
Xu H, Wu L, Zhu J, Navrotsky A (2015) Synthesis, characterization and thermochemistry of Cs-, Rb- and Sr- substituted barium aluminium titanate hollandites. J Nucl Mater 459:70–76. https://doi.org/10.1016/j.jnucmat.2015.01.014
Navi NU, Shneck RZ, Shvareva TY, Kimmel G, Zabicky J, Mintz MH, Navrotsky A (2012) Thermochemistry of (CaxSr1-x)TiO3, (BaxSr1-x)TiO3, and (BaxCa1-x)TiO3 perovskite solid solutions. J Am Ceram Soc 95:1717–1726. https://doi.org/10.1111/j.1551-2916.2012.05137.x
Bailey DJ, Stennett MC, Mason AR, Hyatt NC (2018) Synthesis and characterisation of the hollandite solid solution Ba1.2-xCsxFe2.4-xTi5.6+xO16 for partitioning and conditioning of radiocaesium. J Nucl Mater 503:164–170. https://doi.org/10.1016/j.jnucmat.2018.03.005
Levy MR, Steel BC, Grimes RW (2004) Divalent cation solution in A3+B3+O3 perovskites. Solid State Ionics 175:349–352. https://doi.org/10.1016/j.ssi.2004.02.072
Maddrell E (2013) Hot isostatically pressed waste forms for future nuclear fuel cycles. Chem Eng Res Des 91:735–741. https://doi.org/10.1016/j.cherd.2012.11.004
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J Appl Crystallogr 34:210–213
Strachan DM, Turcotte RP, Barnes BO (1982) MCC-1: a standard leach test for nuclear waste forms. Nucl Technol 56:306–312. https://doi.org/10.13182/NT82-A32859
Feng T, Li L, Lv Z, Li B, Zhang Y, Li G (2019) Temperature-dependent electrical transport behavior and structural evolution in hollandite-type titanium-based oxide. J Am Ceram Soc 102:6741–6750. https://doi.org/10.1111/jace.16520
Wang X, Ma J, Lu X, Fang Z, Li L, Li L, Yang Y (2020) Investigations on the structural evolution and aqueous durability of [CsxBay][Fe3+2y+xTi4+8-2y-x]O16 ceramics for radioactive cesium storage. J Solid State Chem 288:121457. https://doi.org/10.1016/j.jssc.2020.121457
Luxová J, Šulcová P, Trojan M (2008) Study of perovskite compounds. J Therm Anal Calorim 93:823–827. https://doi.org/10.1007/s10973-008-9329-z
Grote R, Zhao M, Shuller-Nickles L, Amoroso J, Gong W, Lilova K, Brinkman KS (2019) Compositional control of tunnel features in hollandite-based ceramics: structure and stability of (Ba, Cs)1.33(Zn, Ti)8O16. J Mater Sci 54:1112–1125. https://doi.org/10.1007/s10853-018-2904-1
Grigor’eva LF, Petrov SA, Sinel’shchikova OY, Gusarov VV (2003) Mechanism of the formation of Ba2Ti9O20-based phases in the course of solid-phase interaction in the BaO-TiO2(ZrO2) and Cs2O-BaO-TiO2(ZrO2) systems. Glass Phys Chem 29:188–193. https://doi.org/10.1023/A:1023415327336
Amoroso JW, Marra J, Dandeneau CS, Brinkman K, Xu Y, Tang M, Maio V, Webb SM, Chiu WKS (2017) Cold crucible induction melter test for crystalline ceramic waste form fabrication: a feasibility assessment. J Nucl Mater 486:283–297. https://doi.org/10.1016/j.jnucmat.2017.01.028
Zhao MY, Russell P, Amoroso J, Misture S, Utlak S, Besmann T, Nickles LS, Brinkman KS (2020) Exploring the links between crystal chemistry, cesium retention, thermochemistry and chemical durability in single-phase (Ba, Cs)1.33(Fe, Ti)8O16 hollandite. J Mater Sci 55:6401–6416. https://doi.org/10.1007/s10853-020-04447-3
Amoroso J, Marra J, Conradson SD, Tang M, Brinkman K (2014) Melt processed single phase hollandite waste forms for nuclear waste immobilization: Ba1.0Cs0.3A2.3Ti5.7O16; A = Cr, Fe. Al J Alloy Compd 584:590–599. https://doi.org/10.1016/j.jallcom.2013.09.087
Xu Y, Wen Y, Grote R, Amoroso J, Nickles LS, Brinkman KS (2016) A-site compositional effects in Ga-doped hollandite materials of the form BaxCsyGa2x+yTi8-2x-yO16: implications for Cs immobilization in crystalline ceramic waste forms. Sci Rep 6:27412. https://doi.org/10.1038/srep27412
Leinekugel-le-Cocq AY, Deniard P, Jobic S, Cerny R, Bart F, Emerich H (2006) Synthesis and characterization of hollandite-type material intended for the specific containment of radioactive cesium. J Solid State Chem 179:3196–3208. https://doi.org/10.1016/j.jssc.2006.05.047
Yang Y, Wang X, Luo S, Yang X, Ma J (2019) Stability studies of [CsxBay][(Al3+, Ti3+)2y+xTi4+8-2y-x]O16 ceramics for radioactive caesium immobilization. Ceram Int 45:7865–7870. https://doi.org/10.1016/j.ceramint.2019.01.095
Bailey DJ, Stennett MC, Hyatt NC (2020) Ba1.2-xCsxM1.2-x/2Ti6.8+x/2O16 (M = Ni, Zn) hollandites for the immobilisation of radiocaesium. MRS Adv 5:55–64. https://doi.org/10.1557/adv.2020.43
Danelska A, Ulkowska U, Socha RP, Szafran M (2013) Surface properties of nanozirconia and their effect on its rheological behaviour and sinterability. J Eur Ceram Soc 33:1875–1883. https://doi.org/10.1016/j.jeurceramsoc.2013.01.019
Tang Y, Shih K (2015) Mechanisms of zinc incorporation in aluminosilicate crystalline structures and the leaching behaviour of product phases. Environ Technol 36:2977–2986. https://doi.org/10.1080/09593330.2014.982715
Gregg DJ, Farzana R, Dayal P, Holmes R, Triani G (2020) Synroc technology: perspectives and current status. J Am Ceram Soc 103:5424–5441. https://doi.org/10.1111/jace.17322
Luca V, Cassidy D, Drabarek E, Murray K, Moubaraki B (2005) Cesium extraction from Cs0.8Ba0.4Ti8O16 hollandite nuclear waste form ceramics in nitric acid solutions. J Mater Res 20:1436–1446. https://doi.org/10.1557/JMR.2005.0204
Kumar SP, Gopal B (2015) Simulated monazite crystalline wasteform La0.4Nd0.1Y0.1Gd0.1Sm0.1Ce0.1Ca0.1(P0.9Mo0.1O4): synthesis, phase stability and chemical durability study. J Nucl Mater 458:224–232. https://doi.org/10.1016/j.jnucmat.2014.12.081
Li WQ, Ding XG, Meng C, Ren CR, Wu HT, Yang H (2018) Phase structure evolution and chemical durability studies of Gd1-xYbxPO4 ceramics for immobilization of minor actinides. J Mater Sci 53:6366–6377. https://doi.org/10.1007/s10853-018-2031-z
Acknowledgements
We sincerely acknowledge the financial support from the National Natural Science Foundation of China (Grant Nos. 41574100, 11705152).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest
Additional information
Handling Editor: M. Grant Norton.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, J., Fang, Z., Yang, X. et al. Investigating hollandite–perovskite composite ceramics as a potential waste form for immobilization of radioactive cesium and strontium. J Mater Sci 56, 9644–9654 (2021). https://doi.org/10.1007/s10853-021-05886-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-05886-2